Sulfur Hazes in Giant Exoplanet Atmospheres: Impacts on Reflected
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Where Are the Distant Worlds? Star Maps
W here Are the Distant Worlds? Star Maps Abo ut the Activity Whe re are the distant worlds in the night sky? Use a star map to find constellations and to identify stars with extrasolar planets. (Northern Hemisphere only, naked eye) Topics Covered • How to find Constellations • Where we have found planets around other stars Participants Adults, teens, families with children 8 years and up If a school/youth group, 10 years and older 1 to 4 participants per map Materials Needed Location and Timing • Current month's Star Map for the Use this activity at a star party on a public (included) dark, clear night. Timing depends only • At least one set Planetary on how long you want to observe. Postcards with Key (included) • A small (red) flashlight • (Optional) Print list of Visible Stars with Planets (included) Included in This Packet Page Detailed Activity Description 2 Helpful Hints 4 Background Information 5 Planetary Postcards 7 Key Planetary Postcards 9 Star Maps 20 Visible Stars With Planets 33 © 2008 Astronomical Society of the Pacific www.astrosociety.org Copies for educational purposes are permitted. Additional astronomy activities can be found here: http://nightsky.jpl.nasa.gov Detailed Activity Description Leader’s Role Participants’ Roles (Anticipated) Introduction: To Ask: Who has heard that scientists have found planets around stars other than our own Sun? How many of these stars might you think have been found? Anyone ever see a star that has planets around it? (our own Sun, some may know of other stars) We can’t see the planets around other stars, but we can see the star. -

On the Detection of Exoplanets Via Radial Velocity Doppler Spectroscopy
The Downtown Review Volume 1 Issue 1 Article 6 January 2015 On the Detection of Exoplanets via Radial Velocity Doppler Spectroscopy Joseph P. Glaser Cleveland State University Follow this and additional works at: https://engagedscholarship.csuohio.edu/tdr Part of the Astrophysics and Astronomy Commons How does access to this work benefit ou?y Let us know! Recommended Citation Glaser, Joseph P.. "On the Detection of Exoplanets via Radial Velocity Doppler Spectroscopy." The Downtown Review. Vol. 1. Iss. 1 (2015) . Available at: https://engagedscholarship.csuohio.edu/tdr/vol1/iss1/6 This Article is brought to you for free and open access by the Student Scholarship at EngagedScholarship@CSU. It has been accepted for inclusion in The Downtown Review by an authorized editor of EngagedScholarship@CSU. For more information, please contact [email protected]. Glaser: Detection of Exoplanets 1 Introduction to Exoplanets For centuries, some of humanity’s greatest minds have pondered over the possibility of other worlds orbiting the uncountable number of stars that exist in the visible universe. The seeds for eventual scientific speculation on the possibility of these "exoplanets" began with the works of a 16th century philosopher, Giordano Bruno. In his modernly celebrated work, On the Infinite Universe & Worlds, Bruno states: "This space we declare to be infinite (...) In it are an infinity of worlds of the same kind as our own." By the time of the European Scientific Revolution, Isaac Newton grew fond of the idea and wrote in his Principia: "If the fixed stars are the centers of similar systems [when compared to the solar system], they will all be constructed according to a similar design and subject to the dominion of One." Due to limitations on observational equipment, the field of exoplanetary systems existed primarily in theory until the late 1980s. -

Star Festival 2016 Parade & Exhibition
Jump!Star Festival 2016 Parade & Exhibition Images from George Ferrandi’s Wherever There is Water light parade for Fleisher Art Memorial 202.234.7103 Capitol Skyline Hotel [email protected] 10 I (Eye) Street SW www.wpadc.org Washington, DC 20024 TABLE OF CONTENTS INTRODUCTION Jump!Star Festival Concept History of the Project Festival Narrative Artist’s Biography COMPONENTS Community Engagement Workshops Light Exhibition Parade/Festival Images from George Ferrandi’s Wherever There is Water light parade for Fleisher Art Memorial INTRODUCTION Images from George Ferrandi’s Wherever There is Water light parade for Fleisher Art Memorial The Jump!Star Festival is a participatory public art project created by artist George Ferrandi that will celebrate the temporal nature of our pole star, the “North Star,” by bringing the local community together with a light parade, music, food, and a culminating exhibition of light sculptures created by local artists. Key Dates Parade/Festival: September 24, 2016 Workshops: May/June/July/August 2016 Exhibition: September 20-25, 2016 History of the Project For the Northern Hemisphere, Polaris, or the “North Star” is the guiding light for 90% of the world’s population, yet this Pole Star, the anchor of our heavens, is not permanent. Because the earth rotates at a slight angle on its axis, there is a slight shift in its rotation so that what we consider our “North Star” changes every few thousand years. When the pyramids were built, for example, there was a different “North Star” reigning over the heavens. Very few realize that our quintessential point of reference and ever reliable, literal “Guiding Light” is a temporary gig. -

Analog SFF, April 2010 by Dell Magazine Authors
Analog SFF, April 2010 by Dell Magazine Authors Dell Magazines www.analogsf.com Copyright ©2010 by Dell Magazines NOTICE: This eBook is licensed to the original purchaser only. Duplication or distribution to any person via email, floppy disk, network, print out, or any other means is a violation of International copyright law and subjects the violator to severe fines and/or imprisonment. This notice overrides the Adobe Reader permissions which are erroneous. This eBook cannot be legally lent or given to others. This eBook is displayed using 100% recycled electrons. 2 Analog SFF, April 2010 by Dell Magazine Authors Cover art by David A. Hardy Cover design by Victoria Green 3 Analog SFF, April 2010 by Dell Magazine Authors CONTENTS Reader's Department: EDITORIAL: THE REST OF THE DATA by Stanley Schmidt Department: BIOLOG: BRENDA COOPER by Richard A. Lovett Novelette: SWORDS AND SADDLES by John G. Hemry Science Fact: WHAT'S IN A KISS?: THE WILD, WONDERFUL WORLD OF PHILEMATOLOG by Richard A. Lovett Reader's Department: IN TIMES TO COME Novelette: SNOWFLAKE KISSES by Holly Hight & Richard A. Lovett Novelette: A SOUND BASIS FOR MISUNDERSTANDING by Carl Frederick Reader's Department: THE ALTERNATE VIEW: TAKEN ON FAITH by Jeffery D. Kooistra Novelette: NOTHIN' BUT BLUE SKIES by Stephen L. Burns Novelette: WHEN WE WERE FAB by Jerry Oltion Novelette: THE PLANET HUNTERS by S.L. Nickerson Novelette: THE ROBOTS' GIRL by Brenda Cooper Reader's Department: THE REFERENCE LIBRARY by Don Sakers Reader's Department: BRASS TACKS Reader's Department: UPCOMING EVENTS by Anthony Lewis * * * * 4 Analog SFF, April 2010 by Dell Magazine Authors Vol. -
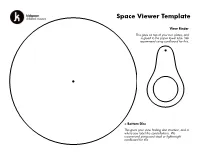
Space Viewer Template
Space Viewer Template View Finder This goes on top of your two plates, and is glued to the paper towel tube. We recommend using cardboard for this. < Bottom Disc This gives your view finding disc structure, and is where you label the constellations. We recommend using card stock or lightweight cardboard for this. Constellation Plate > This disc is home to your constellations, and gets glued to the larger disc. We recommend using paper for this, so the holes are easier to punch. Use the punch guides on the next page to punch holes and add labels to your viewer. Orion Cancer Look for the middle star of Orions When you look at this constellation, look sword, that is an area of brighter nearby for 55 Cancri a star that has light, it’s actually a nebula! The five exoplanets orbiting it. One of those Orion Nebula is a gigantic cloud of plants (55 Cancri e) is a super hot dust and gas, where new stars are planet entirely covered in an ocean of being created. lava! Cygnus Andromeda This constellation is home to the This constellation is very close to the Kepler-186 system, including the Andromeda Galaxy (an enormous planet Kepler-186f. Seen by collection of gas, dust, and billions of NASA's Kepler Space Telescope, stars and solar systems). This spiral this is the first Earth-sized planet galaxy is so bright, you can spot it with discovered that is in"habitable the naked eye! zone" of its star. Ursa Minor Cassiopeia Ursa Minor has two stars known While gazing at Cassiopeia look for with exoplanets orbiting them - the“Pacman Nebula” (It’s official name both are gas giants, that are much is NGC 281). -

Thursday, December 22Nd Swap Meet & Potluck Get-Together Next First
Io – December 2011 p.1 IO - December 2011 Issue 2011-12 PO Box 7264 Eugene Astronomical Society Annual Club Dues $25 Springfield, OR 97475 President: Sam Pitts - 688-7330 www.eugeneastro.org Secretary: Jerry Oltion - 343-4758 Additional Board members: EAS is a proud member of: Jacob Strandlien, Tony Dandurand, John Loper. Next Meeting: Thursday, December 22nd Swap Meet & Potluck Get-Together Our December meeting will be a chance to visit and share a potluck dinner with fellow amateur astronomers, plus swap extra gear for new and exciting equipment from somebody else’s stash. Bring some food to share and any astronomy gear you’d like to sell, trade, or give away. We will have on hand some of the gear that was donated to the club this summer, including mirrors, lenses, blanks, telescope parts, and even entire telescopes. Come check out the bargains and visit with your fellow amateur astronomers in a relaxed evening before Christmas. We also encourage people to bring any new gear or projects they would like to show the rest of the club. The meeting is at 7:00 on December 22nd at EWEB’s Community Room, 500 E. 4th in Eugene. Next First Quarter Fridays: December 2nd and 30th Our November star party was clouded out, along with a good deal of the month afterward. If that sounds familiar, that’s because it is: I changed the date in the previous sentence from October to November and left the rest of the sentence intact. Yes, our autumn weather is predictable. Here’s hoping for a lucky break in the weather for our two December star parties. -

A Spectroscopic Study of Detached Binary Systems Using Precise Radial
A spectroscopic study of detached binary systems using precise radial velocities ———————————————————— A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Astronomy in the University of Canterbury by David J. Ramm —————————– University of Canterbury 2004 Abstract Spectroscopic orbital elements and/or related parameters have been determined for eight bi- nary systems, using radial-velocity measurements that have a typical precision of about 15 m s−1. The orbital periods of these systems range from about 10 days to 26 years, with a median of about 6 years. Orbital solutions were determined for the seven systems with shorter periods. The measurement of the mass ratio of the longest-period system, HD 217166, demonstrates that this important astrophysical quantity can be estimated in a model-free manner with less than 10% of the orbital cycle observed spectroscopically. Single-lined orbital solutions have been derived for five of the binaries. Two of these systems are astrometric binaries: β Ret and ν Oct. The other SB1 systems were 94 Aqr A, θ Ant, and the 10-day system, HD 159656. The preliminary spectroscopic solution for θ Ant (P 18 years), is ∼ the first one derived for this system. The improvement to the precision achieved for the elements of the other four systems was typically between 1–2 orders of magnitude. The very high pre- cision with which the spectroscopic solution for HD 159656 has been measured should allow an investigation into possible apsidal motion in the near future. In addition to the variable radial velocity owing to its orbital motion, the K-giant, ν Oct, has been found to have an additional long-term irregular periodicity, attributed, for the time being, to the rotation of a large surface feature. -

Who Really Discovered the First Exoplanet?
Who Really Discovered the First Exoplanet? Two Swiss astronomers got a well-deserved Nobel for finding an exoplanet, but there’s an intriguing backstory By Josh Winn The year 1995, like 1492, was the dawn of an age of discovery. The new explorers, instead of using seagoing vessels to discover continents, use telescopes to discover planets revolving around distant stars. Thousands of these extrasolar planets, a term usually shortened to “exoplanets,” have been found, including a few potentially Earth- like worlds, along with bizarre objects that bear no resemblance to any of the planets in our solar system. Two of these exoplanet explorers, Michel Mayor and Didier Queloz, were recently awarded half of the Nobel Prize in Physics for the discovery they made in 1995. My colleagues and I are united in our admiration for their pioneering work, and in our pride to be continuing what they began. But there is something peculiar about the Nobel Prize citation. It says: “for the discovery of an exoplanet orbiting a solar-type star.” Shouldn’t it say the first exoplanet? After all, hundreds of astronomers have discovered an exoplanet. I’ve helped find a few. Even high school students and amateur astronomers have discovered them. Did the Nobel Committee make a typographical error? No, they did not, and thereby hangs a tale. Just as it is problematic to decide who discovered America (Christopher Columbus? John Cabot? Leif Erikson? Amerigo Vespucci, whose name is the one that stuck? Those who came on foot from Siberia tens of thousands of years ago?) it is difficult to say who discovered the first exoplanet. -
![Arxiv:2010.00015V3 [Hep-Ph] 26 Apr 2021 Galactic Halo Can Scatter with Exoplanets, Lose Energy, and Gles Are the Same Set of Planets, Without DM Heating](https://docslib.b-cdn.net/cover/2593/arxiv-2010-00015v3-hep-ph-26-apr-2021-galactic-halo-can-scatter-with-exoplanets-lose-energy-and-gles-are-the-same-set-of-planets-without-dm-heating-1662593.webp)
Arxiv:2010.00015V3 [Hep-Ph] 26 Apr 2021 Galactic Halo Can Scatter with Exoplanets, Lose Energy, and Gles Are the Same Set of Planets, Without DM Heating
MIT-CTP/5230 SLAC-PUB-17556 Exoplanets as Sub-GeV Dark Matter Detectors Rebecca K. Leane1, 2, ∗ and Juri Smirnov3, 4, y 1Center for Theoretical Physics, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 2SLAC National Accelerator Laboratory, Stanford University, Stanford, CA 94039, USA 3Center for Cosmology and AstroParticle Physics (CCAPP), The Ohio State University, Columbus, OH 43210, USA 4Department of Physics, The Ohio State University, Columbus, OH 43210, USA (Dated: April 27, 2021) We present exoplanets as new targets to discover Dark Matter (DM). Throughout the Milky Way, DM can scatter, become captured, deposit annihilation energy, and increase the heat flow within exoplanets. We estimate upcoming infrared telescope sensitivity to this scenario, finding actionable discovery or exclusion searches. We find that DM with masses above about an MeV can be probed with exoplanets, with DM-proton and DM-electron scattering cross sections down to about 10−37cm2, stronger than existing limits by up to six orders of magnitude. Supporting evidence of a DM origin can be identified through DM-induced exoplanet heating correlated with Galactic position, and hence DM density. This provides new motivation to measure the temperature of the billions of brown dwarfs, rogue planets, and gas giants peppered throughout our Galaxy. Introduction{Are we alone in the Universe? This ques- Exoplanet Temperatures tion has driven wide-reaching interest in discovering a 104 planet like our own. Regardless of whether or not we ever find alien life, the scientific advances from finding DM Heating and understanding other planets will be enormous. From a particle physics perspective, new celestial bodies pro- vide a vast playground to discover new physics. -

Research and Scientific Support Department 2003 – 2004
COVER 7/11/05 4:55 PM Page 1 SP-1288 SP-1288 Research and Scientific Research Report on the activities of the Support Department Research and Scientific Support Department 2003 – 2004 Contact: ESA Publications Division c/o ESTEC, PO Box 299, 2200 AG Noordwijk, The Netherlands Tel. (31) 71 565 3400 - Fax (31) 71 565 5433 Sec1.qxd 7/11/05 5:09 PM Page 1 SP-1288 June 2005 Report on the activities of the Research and Scientific Support Department 2003 – 2004 Scientific Editor A. Gimenez Sec1.qxd 7/11/05 5:09 PM Page 2 2 ESA SP-1288 Report on the Activities of the Research and Scientific Support Department from 2003 to 2004 ISBN 92-9092-963-4 ISSN 0379-6566 Scientific Editor A. Gimenez Editor A. Wilson Published and distributed by ESA Publications Division Copyright © 2005 European Space Agency Price €30 Sec1.qxd 7/11/05 5:09 PM Page 3 3 CONTENTS 1. Introduction 5 4. Other Activities 95 1.1 Report Overview 5 4.1 Symposia and Workshops organised 95 by RSSD 1.2 The Role, Structure and Staffing of RSSD 5 and SCI-A 4.2 ESA Technology Programmes 101 1.3 Department Outlook 8 4.3 Coordination and Other Supporting 102 Activities 2. Research Activities 11 Annex 1: Manpower Deployment 107 2.1 Introduction 13 2.2 High-Energy Astrophysics 14 Annex 2: Publications 113 (separated into refereed and 2.3 Optical/UV Astrophysics 19 non-refereed literature) 2.4 Infrared/Sub-millimetre Astrophysics 22 2.5 Solar Physics 26 Annex 3: Seminars and Colloquia 149 2.6 Heliospheric Physics/Space Plasma Studies 31 2.7 Comparative Planetology and Astrobiology 35 Annex 4: Acronyms 153 2.8 Minor Bodies 39 2.9 Fundamental Physics 43 2.10 Research Activities in SCI-A 45 3. -

Planet Formation in Binaries 3
Noname manuscript No. (will be inserted by the editor) Planet formation in Binaries P. Thebault · N. Haghighipour the date of receipt and acceptance should be inserted later Abstract Spurred by the discovery of more than 60 exoplanets in multiple systems, binaries have become in recent years one of the main topics in planet formation re- search. Numerous studies have investigated to what extent the presence of a stellar companion can affect the planet formation process. Such studies have implications that can reach beyond the sole context of binaries, as they allow to test certain as- pects of the planet formation scenario by submitting them to extreme environments. We review here the current understanding on this complex problem. We show in par- ticular how each of the different stages of the planet-formation process is affected differently by binary perturbations. We focus especially on the intermediate stage of kilometre-sized planetesimal accretion, which has proven to be the most sensitive to binarity and for which the presence of some exoplanets observed in tight binaries is difficult to explain by in-situ formation following the ”standard” planet-formation scenario. Some tentative solutions to this apparent paradox are presented. The last part of our review presents a thorough description of the problem of planet habit- ability, for which the binary environment creates a complex situation because of the presence of two irradiation sources of varying distance. Keywords Planetary systems · Binary Stars 1 Introduction About half of solar-type stars reside in multiple stellar systems (Raghavan et al., 2010). As a consequence, one of the most generic environments to be considered for studying planet formation should in principle be that of a binary. -

Astronomy and Astrophysics
A Problem book in Astronomy and Astrophysics Compilation of problems from International Olympiad in Astronomy and Astrophysics (2007-2012) Edited by: Aniket Sule on behalf of IOAA international board July 23, 2013 ii Copyright ©2007-2012 International Olympiad on Astronomy and Astrophysics (IOAA). All rights reserved. This compilation can be redistributed or translated freely for educational purpose in a non-commercial manner, with customary acknowledgement of IOAA. Original Problems by: academic committees of IOAAs held at: – Thailand (2007) – Indonesia (2008) – Iran (2009) – China (2010) – Poland (2011) – Brazil (2012) Compiled and Edited by: Aniket Sule Homi Bhabha Centre for Science Education Tata Institute of Fundamental Research V. N. Purav Road, Mankhurd, Mumbai, 400088, INDIA Contact: [email protected] Thank you! Editor would like to thank International Board of International Olympiad on Astronomy and Astrophysics (IOAA) and particularly, president of the board, Prof. Chatief Kunjaya, and general secretary, Prof. Gregorz Stachowaski, for entrusting this task to him. We acknowledge the hard work done by respec- tive year’s problem setters drawn from the host countries in designing the problems and fact that all the host countries of IOAA graciously agreed to permit use of the problems, for this book. All the members of the interna- tional board IOAA are thanked for their support and suggestions. All IOAA participants are thanked for their ingenious solutions for the problems some of which you will see in this book. iv Thank you! A Note about Problems You will find a code in bracket after each problem e.g. (I07 - T20 - C). The first number simply gives the year in which this problem was posed I07 means IOAA2007.