National Oncologic PET Registry
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Ledger and Times, August 27, 1966
Murray State's Digital Commons The Ledger & Times Newspapers 8-27-1966 The Ledger and Times, August 27, 1966 The Ledger and Times Follow this and additional works at: https://digitalcommons.murraystate.edu/tlt Recommended Citation The Ledger and Times, "The Ledger and Times, August 27, 1966" (1966). The Ledger & Times. 5497. https://digitalcommons.murraystate.edu/tlt/5497 This Newspaper is brought to you for free and open access by the Newspapers at Murray State's Digital Commons. It has been accepted for inclusion in The Ledger & Times by an authorized administrator of Murray State's Digital Commons. For more information, please contact [email protected]. • • Selected As A Best All Roland Irennict: C017;rtum1ty Newneper la" The Only Largest Afternoon Daily Circulation In Murray Ai Both In City Calloway County And In County • United Press International In Our 17th Year _Mittray...Ky., Saturday Afternoon, August 27, 1966 Cop,: VOT D(XXVII N 203— 711 Murray High Seen & Heard par LNDAN LOCATICN Marching Band Heavest Raids Of War Around • SPECIFIC WARNING REGARDING INTERROGATIONS MURRAY ceiling Ready 1. YOU HAVE THE RIGHT TO REMAIN SILENT. Hit North Viet Nam; 156 2. ANYTHING YOU SAY CAN AND WILL DE USED AGAINST YOU IN A COURT The Bleck and Gold Marching Band cd Murray Hips School has OF LAW. IS: Hanging pioturea is not one Of Mg been bard at wont getting ready better abilities for their at pankstharte at the 3, You HAVE THE RIGHT TO TALK TO A LAWYER AND HAVE HIM PRESENT Crittenden County plane next Fri- Missions Are Reported WITH Vol.) WHILE YOU ARE CLING QUESTIONED. -

FPGA-Based Data Acquisition System for a Positron Emission Tomography (PET) Scanner Michael Haselman1, Robert Miyaoka2, Thomas K
FPGA-Based Data Acquisition System for a Positron Emission Tomography (PET) Scanner Michael Haselman1, Robert Miyaoka2, Thomas K. Lewellen2, Scott Hauck1 1Department of Electrical Engineering, 2Department of Radiology University of Washington, Seattle, WA {haselman, hauck}@ee.washington.edu, {tkldog, rmiyaoka}@u.washington.edu Abstract: Modern Field Programmable Gate Arrays (FPGAs) are capable of performing complex discrete signal processing algorithms with clock rates of above 100MHz. This combined with FPGAs low expense, ease of use, and selected dedicated hardware make them an ideal technology for a data acquisition system for positron emission tomography (PET) scanner. Our laboratory is producing a high-resolution, small-animal PET scanner that utilizes FPGAs as the core of the front-end electronics. While this scanner uses an Altera ACEX1k and has limited complexity, we are also developing a new set of front-end electronics based on an Altera StratixII. This next generation scanner utilizes many of the features of modern FPGAs to add significant signal processing to produce higher resolution images. One such process we discuss is sub-clock rate pulse timing. We show that timing performed in the FPGA can achieve a resolution that is suitable for small-animal scanners, and will outperform the analog Figure 1. Drawing of a PET scanner ring and attached version given a low enough sampling period for the ADC. electronics. 1. Introduction 2. Positron Emission Tomography The ability to produce images of the inside of a living PET is a medical imaging modality that uses radioactive organism without invasive surgery has been a major decays to measure certain metabolic activities inside living advancement in medicine over the last 100 years. -

Anew Drug Design Strategy in the Liht of Molecular Hybridization Concept
www.ijcrt.org © 2020 IJCRT | Volume 8, Issue 12 December 2020 | ISSN: 2320-2882 “Drug Design strategy and chemical process maximization in the light of Molecular Hybridization Concept.” Subhasis Basu, Ph D Registration No: VB 1198 of 2018-2019. Department Of Chemistry, Visva-Bharati University A Draft Thesis is submitted for the partial fulfilment of PhD in Chemistry Thesis/Degree proceeding. DECLARATION I Certify that a. The Work contained in this thesis is original and has been done by me under the guidance of my supervisor. b. The work has not been submitted to any other Institute for any degree or diploma. c. I have followed the guidelines provided by the Institute in preparing the thesis. d. I have conformed to the norms and guidelines given in the Ethical Code of Conduct of the Institute. e. Whenever I have used materials (data, theoretical analysis, figures and text) from other sources, I have given due credit to them by citing them in the text of the thesis and giving their details in the references. Further, I have taken permission from the copyright owners of the sources, whenever necessary. IJCRT2012039 International Journal of Creative Research Thoughts (IJCRT) www.ijcrt.org 284 www.ijcrt.org © 2020 IJCRT | Volume 8, Issue 12 December 2020 | ISSN: 2320-2882 f. Whenever I have quoted written materials from other sources I have put them under quotation marks and given due credit to the sources by citing them and giving required details in the references. (Subhasis Basu) ACKNOWLEDGEMENT This preface is to extend an appreciation to all those individuals who with their generous co- operation guided us in every aspect to make this design and drawing successful. -

PET--The History Behind the Technology
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Senior Thesis Projects, 1993-2002 College Scholars 2002 PET--The History Behind the Technology Michael A. Steiner Follow this and additional works at: https://trace.tennessee.edu/utk_interstp2 Recommended Citation Steiner, Michael A., "PET--The History Behind the Technology" (2002). Senior Thesis Projects, 1993-2002. https://trace.tennessee.edu/utk_interstp2/107 This Project is brought to you for free and open access by the College Scholars at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Senior Thesis Projects, 1993-2002 by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. PET - The history Behind the Technology College Scholars Senior Project Michael A. Steiner May 6,2002 Committee Members: Dr. George Kabalka Dr. Gary T. Smith Raymond Trotta Brief Overview Positron Emission Tomography, commonly known by the acronym PET, is a branch of nuclear medicine that has come to be an important imaging diagnostic in the medical field. PET relies on the use ofradioactive isotopes to visualize physiological processes ofthe body. An isotope of an element has the same number of protons as its counterpart but di ffers in the nun1ber ofneutrons. The difference in neutrons gives the isotope a different mass number and can also provide instability to the element or molecule derived from the isotope. There are two fundamental differences between traditional nuclear medicine and PET. First, the radiotracers that are utilized in PET emit positrons. Also, there is difference in the process by which data is acquired and reconstructed into an image. -

Future Supply of Medical Radioisotopes for the UK Report 2014
Future Supply of Medical Radioisotopes for the UK Report 2014 Report prepared by: British Nuclear Medicine Society and Science & Technology Facilities Council. December 2014 1 Preface Technetium-99m (99mTc) is the principal radioisotope used in medical diagnostics worldwide. Current estimates are that 99mTc is used in 30 million procedures per year globally and accounts for 80 to 85% of all diagnostic investigations using Nuclear Medicine techniques. Its 6-hour physical half-life and the 140 keV photopeak makes it ideally suited to medical imaging using conventional gamma cameras. 99mTc is derived from its parent element molybdenum-99 (99Mo) that has a physical half-life of 66 hours. At present 99Mo is derived almost exclusively from the fission of uranium-235 targets (using primarily highly-enriched uranium) irradiated in a small number of research nuclear reactors. A global shortage of 99Mo in 2008/09 exposed vulnerabilities in the supply chain of medical radioisotopes. In response, and at the request of member states, the Organization of Economic Co-operation and Development (OECD) Nuclear Energy Agency (NEA) assembled a response team and in April 2009 formed a High-Level Group on the security of supply of Medical Radioisotopes (HLG-MR). The HLG-MR terms of reference are: to review the total 99Mo supply chain from uranium procurement for targets to patient delivery; to identify weak points and issues in the supply chain in the short, medium and long-term; to recommend options to address vulnerabilities to help ensure stable and secure supply of radioisotopes. The UK has no research nuclear reactors and relies on the importation of 99Mo and other medical radioisotopes (e.g. -

Preliminary Program
PRELIMINARY PROGRAM 55th Annual Meeting of the Health Physics Society 22nd Biennial Campus Radiation Safety Officers Meeting (American Conference of Radiological Safety) 27 June - 1 July 2010 Salt Palace Convention Center Salt Lake City, Utah Key Dates Current Events/Works-In-Progress Deadline . .28 May Social/Technical Preregistration Deadline . .31 May HPS Annual Meeting Preregistration Deadline . 31 May PEP Preregistration Deadline . .31 May Hotel Registration Deadline . 5 June AAHP Courses . 26 June Professional Enrichment Program . 27 June - 1 July HPS 55th Annual Meeting . 27 June - 1 July American Board of Health Physics Written Exam . 28 June Registration Hours and Location Registration at the Salt Palace Convention Center - Foyer of Exhibit Hall A Saturday, 26 June . 2:00 - 5:00 pm Sunday, 27 June . 10:00 am - 5:00 pm Monday, 28 June . 8:00 am - 4:00 pm Tuesday, 29 June . 8:00 am - 4:00 pm Wednesday, 30 June . 8:00 am - 4:00 pm Thursday, 1 July . 8:00 - 11:00 am Saturday Saturday AAHP Courses will take place in the Hilton Hotel Sunday - Thursday All PEPs, CELs and Sessions will be at the Salt Palace Convention Center HPS Secretariat 1313 Dolley Madison Blvd. Suite 402 McLean, VA22101 (703) 790-1745; FAX: (703) 790-2672 Email: [email protected]; Website: www.hps.org 1 Table of Contents Important Events . 6 General Information . 7 Hotel Reservation Information . 7 Tours and Events Listing . 9 Scientific Program . 12 Placement Information . 32 AAHP Courses . 33 Professional Enrichment Program . 34 Continuing Education Lecture Abstracts . 46 Annual Meeting Registration Form . 48-49 CURRENT EVENTS/WORKS-IN-PROGRESS The submission form for the Current Events/Works-in-Progress poster session is on the Health Physics Society Website at www .hps .org under the Salt Lake City Annual Meeting section . -
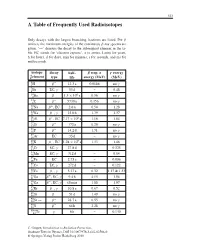
A Table of Frequently Used Radioisotopes
323 A Table of Frequently Used Radioisotopes Only decays with the largest branching fractions are listed. For β emitters the maximum energies of the continuous β-ray spectra are given. ‘→’ denotes the decay to the subsequent element in the ta- ble. EC stands for ‘electron capture’, a (= annus, Latin) for years, h for hours, d for days, min for minutes, s for seconds, and ms for milliseconds. isotope decay half- β resp. α γ energy A Z element type life energy (MeV) (MeV) 3 β− . γ 1H 12 3a 0.0186 no 7 γ 4Be EC, 53 d – 0.48 10 β− . × 6 γ 4Be 1 5 10 a 0.56 no 14 β− γ 6C 5730 a 0.156 no 22 β+ . 11Na ,EC 2 6a 0.54 1.28 24 β− γ . 11Na , 15 0h 1.39 1.37 26 β+ . × 5 13Al ,EC 7 17 10 a 1.16 1.84 32 β− γ 14Si 172 a 0.20 no 32 β− . γ 15P 14 2d 1.71 no 37 γ 18Ar EC 35 d – no 40 β− . × 9 19K ,EC 1 28 10 a 1.33 1.46 51 γ . 24Cr EC, 27 8d – 0.325 54 γ 25Mn EC, 312 d – 0.84 55 . 26Fe EC 2 73 a – 0.006 57 γ 27Co EC, 272 d – 0.122 60 β− γ . 27Co , 5 27 a 0.32 1.17 & 1.33 66 β+ γ . 31Ga , EC, 9 4h 4.15 1.04 68 β− γ 31Ga , EC, 68 min 1.88 1.07 85 β− γ . -

Trends in Radiopharmaceuticals
ISTR–2019 International Symposium on Trends in Radiopharmaceuticals 28 October–1 November 2019 Vienna, Austria Programme & Abstracts ISTR–2019 Organized by Colophon This book has been assembled from the abstract sources submitted by the contributing authors via the Indico conference management platform. Layout, editing, and typesetting of the book, was done by Ms. Julia S. Vera Araujo from the Radioisotope Products and Radiation Technology section, IAEA, Vienna, Austria. This book is PDF hyperlinked: activating coloured text will, in general, move you throughout the book, or link to external resources on the web. ISTR–2019 INTRODUCTION Progress in nuclear medicine has been always tightly linked to the development of new radiopharmaceuticals and efficient production of relevant radioisotopes. The use of radiopharmaceuticals is an important tool for better understanding of human diseases and developing effective treatments. The availability of new radioisotopes and radiopharmaceuticals may generate unprecedented solutions to clinical problems by providing better diagnosis and more efficient therapies. Impressive progress has been made recently in the radioisotope production technologies owing to the introduction of high-energy and high-current cyclotrons and the growing interest in the use of linear accelerators for radioisotope production. This has allowed broader access to several new radionuclides, including gallium-68, copper-64 and zirconium-89. Development of high-power electron linacs resulted in availability of theranostic beta emitters such as scandium-47 and copper-67. Alternative, accelerator-based production methods of technetium-99m, which remains the most widely used diagnostic radionuclide, are also being developed using both electron and proton accelerators. Special attention has been recently given to α-emitting radionuclides for in-vivo therapy. -

Applications of Radiation Overview
Applications of Radiation Overview • General applications by radiation type • Radiography - process • Medical Research • Medical Applications •Space Alpha Radiation •Highly ionizing • Removes Static Charge Alpha Particle Static Charge Uses of Alpha Radiation • Pacemakers (Older models) • Airplanes • Copy Machines •Smoke Detectors •Space exploration Beta Radiation • Small electron particle •More penetrating than alpha e- Beta Radiation is used in thickness gauging The thicker the material, the less radiation will pass through the material. Gauging is used to: • Measure and control thickness of paper, plastic, and aluminum. • Measure the amount of glue placed on a postage stamp • Measure the amount of air whipped into ice cream. • Measure the density of the road during construction. Back Scattering Detector Gamma Radiation A penetrating wave Uses for Gamma radiation . Food irradiation . Sterilization of medical equipment . Creation of different varieties of flowers . Inspect bridges, vessel welds and Statue Of Liberty. Nitrogen-14 Carbon-14 Cycle http://science.howstuffworks.com/ environmental/earth/geology/carb on-141.htm What were original uses of mysterious rays? • Becquerel’s discovery • Roentgen X-ray of Wife’s hand •Marie Curie – WWI – x-ray unit Early X-ray Source: http://www.uihealthcare.com/depts/ medmuseum/galleryexhibits/collecti ngfrompast/xray/xray.html Radiographs X-ray • Radiograph - radiation energy passes through object Photo-Film • Autoradiograph - use radiation from object itself (0.025 eV) Compare Different Materials -

SNMMI Newsline
NEWSLINE SNMMI Hosts FDA Workshop n February 21, SNMMI and the U.S. Food and Drug Administration (FDA), Medical Imaging Technology OAlliance, and World Molecular Imaging Society hosted ‘‘PET Drugs: A Workshop on Inspections Manage- ment and Regulatory Issues,’’ at the FDA White Oak Con- ference Center in Silver Spring, MD. The purpose of the workshop was to provide a forum for exchange of information and perspectives on the regulatory and compliance frame- work for PET drug manufacturing and thereby improve global understanding of PET drug manufacturing. The work- shop organizers included Sue Bunning, MA (MITA), Dalton Clark (SNMMI), Steve Mattmuller, MS, RPh (Kettering Medical Center), Sally Schwarz, MS (Washington Univer- sity), Henry VanBrocklin, PhD (University of California San Francisco), and Steve Zigler, PhD (PETNET Solutions). The At the Workshop on PET Drug Manufacturing: Sue Bunning, MS, Steve Zigler, PhD, Sally Shwarz, MS, Steve Mattmuller, workshop was attended by approximately 150 members of MS, RPh. the PET community and the FDA. Among the specific goals and objectives of the 1-day meeting were to: discuss regulatory compliance for devel- focused on lifecycle management of PET drug applications, opment and manufacturing of PET drugs and pathways for including management of PET drug applications (NDA drug applications, application maintenance, and inspections or ANDA), and PET community perspectives on PET drug based on Code of Federal Regulations Part 212 (Current application, with a follow-up discussion and question Good Manufacturing Practice [cGMP] for Positron Emission period. After lunch, the third session looked at chemistry Tomography Drugs); share perspectives from industry, acade- and product quality assurance, including the microbiolog- mia, investigators, and regulators on inspection findings and ical regulatory perspective; product quality and sterility trends; and provide information on the management of Part 212 assurance; and chemistry and product quality assurance. -

Medical Applications of Nuclear Physics 1149
CHAPTER 32 | MEDICAL APPLICATIONS OF NUCLEAR PHYSICS 1149 32 MEDICAL APPLICATIONS OF NUCLEAR PHYSICS Figure 32.1 Tori Randall, Ph.D., curator for the Department of Physical Anthropology at the San Diego Museum of Man, prepares a 550-year-old Peruvian child mummy for a CT scan at Naval Medical Center San Diego. (credit: U.S. Navy photo by Mass Communication Specialist 3rd Class Samantha A. Lewis) Learning Objectives 32.1. Medical Imaging and Diagnostics • Explain the working principle behind an anger camera. • Describe the SPECT and PET imaging techniques. 32.2. Biological Effects of Ionizing Radiation • Define various units of radiation. • Describe RBE. 32.3. Therapeutic Uses of Ionizing Radiation • Explain the concept of radiotherapy and list typical doses for cancer therapy. 32.4. Food Irradiation • Define food irradiation low dose, and free radicals. 32.5. Fusion • Define nuclear fusion. • Discuss processes to achieve practical fusion energy generation. 32.6. Fission • Define nuclear fission. • Discuss how fission fuel reacts and describe what it produces. • Describe controlled and uncontrolled chain reactions. 32.7. Nuclear Weapons • Discuss different types of fission and thermonuclear bombs. • Explain the ill effects of nuclear explosion. 1150 CHAPTER 32 | MEDICAL APPLICATIONS OF NUCLEAR PHYSICS Introduction to Applications of Nuclear Physics Applications of nuclear physics have become an integral part of modern life. From the bone scan that detects a cancer to the radioiodine treatment that cures another, nuclear radiation has diagnostic and therapeutic effects on medicine. From the fission power reactor to the hope of controlled fusion, nuclear energy is now commonplace and is a part of our plans for the future. -

R&D and Innovation Needs for Decommissioning of Nuclear
Radioactive Waste Management 2014 R&D and Innovation Needs for Decommissioning Nuclear Facilities NEA Radioactive Waste Management R&D and Innovation Needs for Decommissioning of Nuclear Facilities © OECD 2014 NEA No. 7191 NUCLEAR ENERGY AGENCY ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT ORGANISATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT The OECD is a unique forum where the governments of 34 democracies work together to address the economic, social and environmental challenges of globalisation. The OECD is also at the forefront of efforts to understand and to help governments respond to new developments and concerns, such as corporate governance, the information economy and the challenges of an ageing population. The Organisation provides a setting where governments can compare policy experiences, seek answers to common problems, identify good practice and work to co-ordinate domestic and international policies. The OECD member countries are: Australia, Austria, Belgium, Canada, Chile, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Luxembourg, Mexico, the Netherlands, New Zealand, Norway, Poland, Portugal, the Republic of Korea, the Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, the United Kingdom and the United States. The European Commission takes part in the work of the OECD. OECD Publishing disseminates widely the results of the Organisation’s statistics gathering and research on economic, social and environmental issues, as well