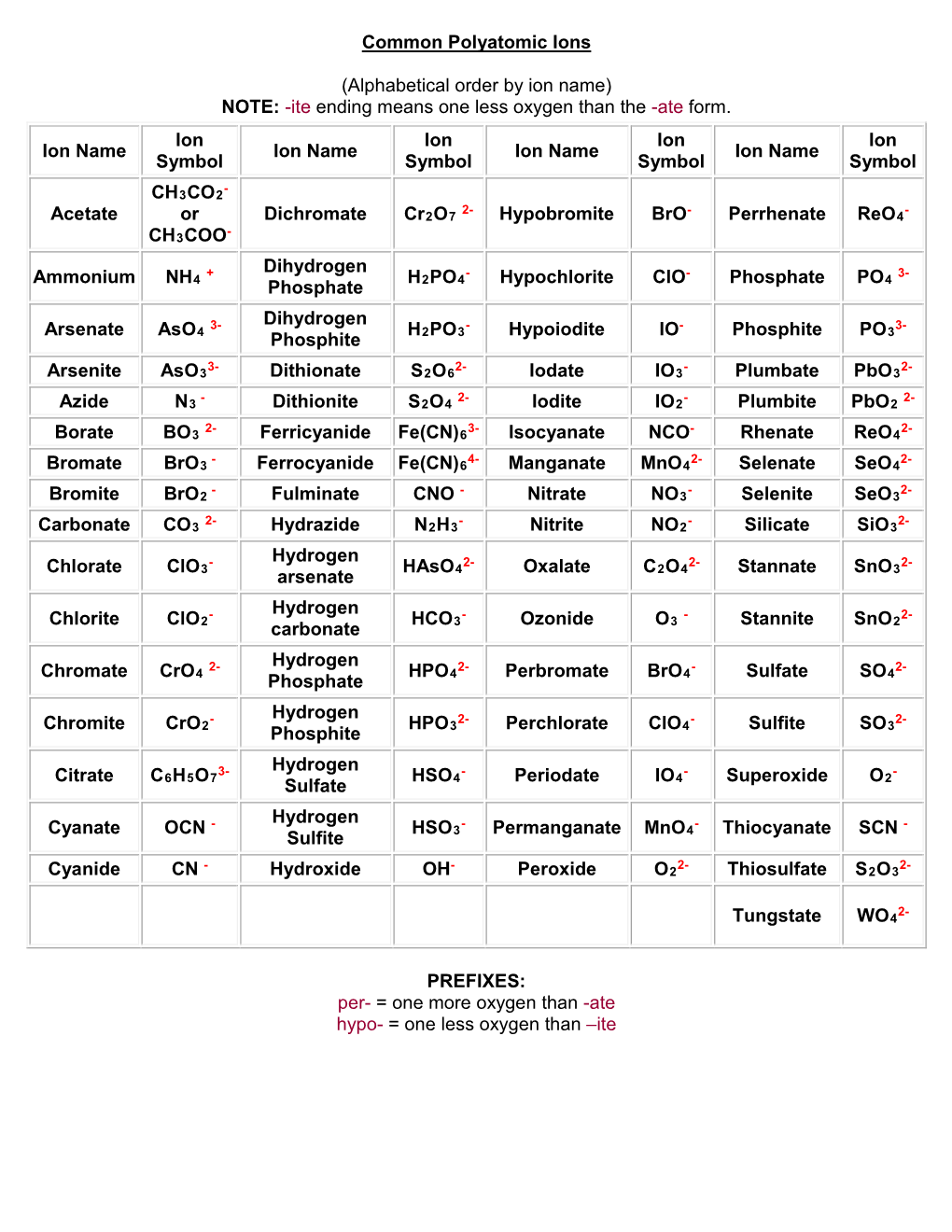
Common Polyatomic Ions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry Grade Level 10 Units 1-15
COPPELL ISD SUBJECT YEAR AT A GLANCE GRADE HEMISTRY UNITS C LEVEL 1-15 10 Program Transfer Goals ● Ask questions, recognize and define problems, and propose solutions. ● Safely and ethically collect, analyze, and evaluate appropriate data. ● Utilize, create, and analyze models to understand the world. ● Make valid claims and informed decisions based on scientific evidence. ● Effectively communicate scientific reasoning to a target audience. PACING 1st 9 Weeks 2nd 9 Weeks 3rd 9 Weeks 4th 9 Weeks Unit 1 Unit 2 Unit 3 Unit 4 Unit 5 Unit 6 Unit Unit Unit Unit Unit Unit Unit Unit Unit 7 8 9 10 11 12 13 14 15 1.5 wks 2 wks 1.5 wks 2 wks 3 wks 5.5 wks 1.5 2 2.5 2 wks 2 2 2 wks 1.5 1.5 wks wks wks wks wks wks wks Assurances for a Guaranteed and Viable Curriculum Adherence to this scope and sequence affords every member of the learning community clarity on the knowledge and skills on which each learner should demonstrate proficiency. In order to deliver a guaranteed and viable curriculum, our team commits to and ensures the following understandings: Shared Accountability: Responding -

Prebiological Evolution and the Metabolic Origins of Life
Prebiological Evolution and the Andrew J. Pratt* Metabolic Origins of Life University of Canterbury Keywords Abiogenesis, origin of life, metabolism, hydrothermal, iron Abstract The chemoton model of cells posits three subsystems: metabolism, compartmentalization, and information. A specific model for the prebiological evolution of a reproducing system with rudimentary versions of these three interdependent subsystems is presented. This is based on the initial emergence and reproduction of autocatalytic networks in hydrothermal microcompartments containing iron sulfide. The driving force for life was catalysis of the dissipation of the intrinsic redox gradient of the planet. The codependence of life on iron and phosphate provides chemical constraints on the ordering of prebiological evolution. The initial protometabolism was based on positive feedback loops associated with in situ carbon fixation in which the initial protometabolites modified the catalytic capacity and mobility of metal-based catalysts, especially iron-sulfur centers. A number of selection mechanisms, including catalytic efficiency and specificity, hydrolytic stability, and selective solubilization, are proposed as key determinants for autocatalytic reproduction exploited in protometabolic evolution. This evolutionary process led from autocatalytic networks within preexisting compartments to discrete, reproducing, mobile vesicular protocells with the capacity to use soluble sugar phosphates and hence the opportunity to develop nucleic acids. Fidelity of information transfer in the reproduction of these increasingly complex autocatalytic networks is a key selection pressure in prebiological evolution that eventually leads to the selection of nucleic acids as a digital information subsystem and hence the emergence of fully functional chemotons capable of Darwinian evolution. 1 Introduction: Chemoton Subsystems and Evolutionary Pathways Living cells are autocatalytic entities that harness redox energy via the selective catalysis of biochemical transformations. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Sodium Periodate Solution (SP7469-G)
Page: 1/9 Safety Data Sheet according to OSHA HCS (29CFR 1910.1200) and WHMIS 2015 Regulations Revision: July 09, 2020 1 Identification · Product identifier · Trade name: Sodium Periodate Solution · Product code: SP7469-G · Recommended use and restriction on use · Recommended use: Laboratory chemicals · Restrictions on use: No relevant information available. · Details of the supplier of the Safety Data Sheet · Manufacturer/Supplier: AquaPhoenix Scientific, Inc. 860 Gitts Run Road Hanover, PA 17331 USA Tel +1 (717)632-1291 Toll-Free: (866)632-1291 [email protected] · Distributor: AquaPhoenix Scientific 860 Gitts Run Road, Hanover, PA 17331 (717) 632-1291 · Emergency telephone number: ChemTel Inc. (800)255-3924 (North America) +1 (813)248-0585 (International) 2 Hazard(s) identification · Classification of the substance or mixture Skin Irrit. 2 H315 Causes skin irritation. Eye Irrit. 2A H319 Causes serious eye irritation. STOT RE 1 H372 Causes damage to the thyroid through prolonged or repeated exposure. · Label elements · GHS label elements The product is classified and labeled according to the Globally Harmonized System (GHS). · Hazard pictograms: GHS07 GHS08 · Signal word: Danger · Hazard statements: H315 Causes skin irritation. H319 Causes serious eye irritation. H372 Causes damage to the thyroid through prolonged or repeated exposure. · Precautionary statements: P260 Do not breathe mist/vapors/spray. P264 Wash thoroughly after handling. (Cont'd. on page 2) 50.1.3 Page: 2/9 Safety Data Sheet according to OSHA HCS (29CFR 1910.1200) and WHMIS 2015 Regulations Revision: July 09, 2020 Trade name: Sodium Periodate Solution (Cont'd. of page 1) P270 Do not eat, drink or smoke when using this product. -

Production of Dialdehyde Cellulose and Periodate Regeneration: Towards Feasible Oxidation Processes
Production of Dialdehyde Cellulose and Periodate Regeneration: Towards feasible oxidation processes Produktion av dialdehydcellulosa och återgenerering av perjodat: Mot möjliga oxidationsprocesser Elisabeth Höglund Department of Engineering and Chemical Sciences Chemistry 30 hp Supervisors: Susanne Hansson, Stora Enso & Gunilla Carlsson, Karlstad University Examinator: Thomas Nilsson 2015-09-25 ABSTRACT Cellulose is an attractive raw material that has lately become more interesting thanks to its degradability and renewability and the environmental awareness of our society. With the intention to find new material properties and applications, studies on cellulose derivatization have increased. Dialdehyde cellulose (DAC) is a derivative that is produced by selective cleavage of the C2-C3 bond in an anhydroglucose unit in the cellulose chain, utilizing sodium periodate (NaIO4) that works as a strong oxidant. At a fixed temperature, the reaction time as well as the amount of added periodate affect the resulting aldehyde content. DAC has shown to have promising properties, and by disintegrating the dialdehyde fibers into fibrils, thin films with extraordinary oxygen barrier at high humidity can be achieved. Normally, barrier properties of polysccharide films deteriorate at higher humidity due to their hygroscopic character. This DAC barrier could therefore be a potential environmentally-friendly replacement for aluminum which is utilized in many food packages today. The aim of this study was to investigate the possibilities to produce dialdehyde cellulose at an industrial level, where the regeneration of consumed periodate plays a significant role to obtain a feasible process. A screening of the periodate oxidation of cellulose containing seven experiments was conducted by employing the program MODDE for experimental design. -

Oregon Department of Human Services HEALTH EFFECTS INFORMATION
Oregon Department of Human Services Office of Environmental Public Health (503) 731-4030 Emergency 800 NE Oregon Street #604 (971) 673-0405 Portland, OR 97232-2162 (971) 673-0457 FAX (971) 673-0372 TTY-Nonvoice TECHNICAL BULLETIN HEALTH EFFECTS INFORMATION Prepared by: Department of Human Services ENVIRONMENTAL TOXICOLOGY SECTION Office of Environmental Public Health OCTOBER, 1998 CALCIUM CARBONATE "lime, limewater” For More Information Contact: Environmental Toxicology Section (971) 673-0440 Drinking Water Section (971) 673-0405 Technical Bulletin - Health Effects Information CALCIUM CARBONATE, "lime, limewater@ Page 2 SYNONYMS: Lime, ground limestone, dolomite, sugar lime, oyster shell, coral shell, marble dust, calcite, whiting, marl dust, putty dust CHEMICAL AND PHYSICAL PROPERTIES: - Molecular Formula: CaCO3 - White solid, crystals or powder, may draw moisture from the air and become damp on exposure - Odorless, chalky, flat, sweetish flavor (Do not confuse with "anhydrous lime" which is a special form of calcium hydroxide, an extremely caustic, dangerous product. Direct contact with it is immediately injurious to skin, eyes, intestinal tract and respiratory system.) WHERE DOES CALCIUM CARBONATE COME FROM? Calcium carbonate can be mined from the earth in solid form or it may be extracted from seawater or other brines by industrial processes. Natural shells, bones and chalk are composed predominantly of calcium carbonate. WHAT ARE THE PRINCIPLE USES OF CALCIUM CARBONATE? Calcium carbonate is an important ingredient of many household products. It is used as a whitening agent in paints, soaps, art products, paper, polishes, putty products and cement. It is used as a filler and whitener in many cosmetic products including mouth washes, creams, pastes, powders and lotions. -

Chapter 10 – Chemical Reactions Notes
Chapter 8 – Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if a chemical reaction has occurred (recap): Temperature changes that can’t be accounted for. o Exothermic reactions give off energy (as in fire). o Endothermic reactions absorb energy (as in a cold pack). Spontaneous color change. o This happens when things rust, when they rot, and when they burn. Appearance of a solid when two liquids are mixed. o This solid is called a precipitate. Formation of a gas / bubbling, as when vinegar and baking soda are mixed. Overall, the most important thing to remember is that a chemical reaction produces a whole new chemical compound. Just changing the way that something looks (breaking, melting, dissolving, etc) isn’t enough to qualify something as a chemical reaction! Balancing Equations Notes: Things to keep in mind when looking at the recipes for chemical reactions: 1) The stuff before the arrow is referred to as the “reactants” or “reagents”, and the stuff after the arrow is called the “products.” 2) The number of atoms of each element is the same on both sides of the arrow. Even though there may be different numbers of molecules, the number of atoms of each element needs to remain the same to obey the law of conservation of mass. 3) The numbers in front of the formulas tell you how many molecules or moles of each chemical are involved in the reaction. 4) Equations are nothing more than chemical recipes. -

Chemistry - B.S
Chemistry - B.S. College of (Biochemistry Option) Arts and Sciences The Department of Chemistry offers the Bachelor of Science degree for students who Graduation Composition and Communication Requirement intend to become professional chemists or do graduate work in chemistry or a closely (GCCR) related discipline. There are three options in the B.S. program: a traditional track WRD 310 Writing in the Natural Sciences ............................................................. 3 covering all the major areas of chemistry, an option that emphasizes biochemistry and an option in materials chemistry. The Biochemistry and Traditional Options are Graduation Composition and Communication certified by the American Chemical Society. A Bachelor of Arts degree program is Requirement hours (GCCR) .................................................................... 3 offered as well for students who want greater flexibility in the selection of courses to perhaps pursue more diverse degree options, including dual and double majors. For College Requirements all majors CHE 109 and CHE 110 have been defined as equivalent to CHE 105. The I. Foreign Language (placement exam recommended) ................................... 0-14 Department also offers the Master of Science and the Doctor of Philosophy degree. II. Disciplinary Requirements a. Natural Science (completed by Major Requirements) 128 hours b. Social Science ......................................................................................... 3 Any student earning a Bachelor of Science (BS) -

Sodium Chlorite Neutralization
® Basic Chemicals Sodium Chlorite Neutralization Introduction that this reaction is exothermic and liberates a If sodium chlorite is spilled or becomes a waste, significant amount of heat (H). it must be disposed of in accordance with local, state, and Federal regulations by a NPDES NaClO2 + 2Na2SO3 2Na2SO4 + NaCl permitted out-fall or in a permitted hazardous 90.45g + 2(126.04g) 2(142.04g) + 58.44g waste treatment, storage, and disposal facility. H = -168 kcal/mole NaClO2 Due to the reactivity of sodium chlorite, neutralization for disposal purposes should be For example, when starting with a 5% NaClO2 avoided whenever possible. Where permitted, solution, the heat generated from this reaction the preferred method for handling sodium could theoretically raise the temperature of the chlorite spills and waste is by dilution, as solution by 81C (146F). Adequate dilution, discussed in the OxyChem Safety Data Sheet thorough mixing and a slow rate of reaction are (SDS) for sodium chlorite in Section 6, important factors in controlling the temperature (Accidental Release Measures). Sodium chlorite increase (T). neutralization procedures must be carried out only by properly trained personnel wearing Procedure appropriate protective equipment. The complete neutralization procedure involves three sequential steps: dilution, chlorite Reaction Considerations reduction, and alkali neutralization. The dilution If a specific situation requires sodium chlorite to step lowers the strength of the sodium chlorite be neutralized, the chlorite must first be reduced solution to 5% or less; the reduction step reacts by a reaction with sodium sulfite. The use of the diluted chlorite solution with sodium sulfite to sodium sulfite is recommended over other produce a sulfate solution, and the neutralization reducing agents such as sodium thiosulfate step reduces the pH of the alkaline sulfate (Na2S2O3), sodium bisulfite (NaHSO3), and solution from approximately 12 to 4-5. -

Primary-Explosives
Improvised Primary Explosives © 1998 Dirk Goldmann No part of the added copyrighted parts (except brief passages that a reviewer may quote in a review) may be reproduced in any form unless the reproduced material includes the following two sentences: The copyrighted material may be reproduced without obtaining permission from anyone, provided: (1) all copyrighted material is reproduced full-scale. WARNING! Explosives are danegerous. In most countries it's forbidden to make them. Use your mind. You as an explosives expert should know that. 2 CONTENTS Primary Explosives ACETONE PEROXIDE 4 DDNP/DINOL 6 DOUBLE SALTS 7 HMTD 9 LEAD AZIDE 11 LEAD PICRATE 13 MEKAP 14 MERCURY FULMINATE 15 "MILK BOOSTER" 16 NITROMANNITE 17 SODIUM AZIDE 19 TACC 20 Exotic and Friction Primers LEAD NITROANILATE 22 NITROGEN SULFIDE 24 NITROSOGUANIDINE 25 TETRACENE 27 CHLORATE-FRICTION PRIMERS 28 CHLORATE-TRIMERCURY-ACETYLIDE 29 TRIHYDRAZINE-ZINC (II) NITRATE 29 Fun and Touch Explosives CHLORATE IMPACT EXPLOSIVES 31 COPPER ACETYLIDE 32 DIAMMINESILVER II CHLORATE 33 FULMINATING COPPER 33 FULMINATING GOLD 34 FULMINATING MERCURY 35 FULMINATING SILVER 35 NITROGEN TRICHLORIDE 36 NITROGEN TRIIODIDE 37 SILVER ACETYLIDE 38 SILVER FULMINATE 38 "YELLOW POWDER" 40 Latest Additions 41 End 3 PRIMARY EXPLOSIVES ACETONE PEROXIDE Synonyms: tricycloacetone peroxide, acetontriperoxide, peroxyacetone, acetone hydrogen explosive FORMULA: C9H18O6 VoD: 3570 m/s @ 0.92 g/cc. 5300 m/s @ 1.18 g/cc. EQUIVALENCE: 1 gram = No. 8 cap .75 g. = No. 6 cap SENSITIVITY: Very sensitive to friction, flame and shock; burns violently and can detonate even in small amounts when dry. DRAWBACKS: in 10 days at room temp. 50 % sublimates; it is best made immediately before use. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

NACE Bromine Chemistry Review Paper
25 YEARS OF BROMINE CHEMISTRY IN INDUSTRIAL WATER SYSTEMS: A REVIEW Christopher J. Nalepa Albemarle Corporation P.O. Box 14799 Baton Rouge, LA 70898 ABSTRACT Bromine chemistry is used to great advantage in nature for fouling control by a number of sessile marine organisms such as sponges, seaweeds, and bryozoans. Such organisms produce small quantities of brominated organic compounds that effectively help keep their surfaces clean of problem bacteria, fungi, and algae. For over two decades, bromine chemistry has been used to similar advantage in the treatment of industrial water systems. The past several years in particular has seen the development of several diverse bromine product forms – one-drum stabilized bromine liquids, all-bromine hydantoin solids, and pumpable gels. The purpose of this paper is to review the development of bromine chemistry in industrial water treatment, discuss characteristics of the new product forms, and speculate on future developments. Keywords: Oxidizing biocide, bleach, bromine, bromine chemistry, sodium hypobromite, activated sodium bromide, Bromochlorodimethylhydantoin, Bromochloromethyethylhydantoin, Dibromodi- methylhydantoin,, BCDMH, BCMEH, DBDMH, stabilized bromine chloride, stabilized hypobromite INTRODUCTION Sessile marine organisms generate metabolites to ward off predators and deter attachment of potential micro- and macrofoulants. Sponges, algae, and bryozoans for example, produce a rich variety of bromine-containing compounds that exhibit antifoulant properties (Fig. 1).1,2,3 Scientists are actively studying these organisms to understand how they maintain surfaces that are relatively clean and slime- free.4 Brominated furanones isolated from the red algae Delisea pulchra, for example, have been found to interfere with the chemical signals (acylated homoserine lactones) that bacteria use to communicate with one another to produce biofilms.5,6 This work may eventually lead to more effective control of microorganisms in a number of industries such as industrial water treatment, oil and gas production, health care, etc.