Miller–Urey Experiment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Production of Amino Acids Under Possible Primitive Earth Conditions
A Production of Amino Acids Under Possible Primitive Earth Conditions Stanley L. Miller Methods and Logic - 2/24/15 Outline for today’s class • The origin of life • Stanley L. Miller and Harold Urey • Background • Landmark Paper • Landmark Experiment • Subsequent Studies There are many theories regarding the origin of life • Theory of spontaneous generation: living organisms can arise suddenly and spontaneously from any kind of non-living matter • Aristotle, ancient Egyptians • Popular until 1600s when it was disproved due to various experiments • Fransisco Redi (1665) http://www.tutorvista.com/content/b iology/biology-iii/origin-life/origin- • Louis Pasteur (1864) life-theories.php# http://bekarice.com/college-spontaneous-generation/ There are many theories regarding the origin of life • Cozmozoic theory (parpermia): life reached Earth from other heavenly bodies such as meteorites, in the form of highly resistance spores of some organisms • Richter (1865) • Arrhenius (1908) • Overall lack of evidence Wikipedia • Living matter cannot survive the extreme cold, dryness and ultra-violet radiation from the sun required to be crossed for reaching the earth. There are many theories regarding the origin of life • Theory of chemical evolution: Origin of life on earth is the result of a slow and gradual process of chemical evolution that probably occurred about 4 billion years ago • Oparin (1923) • Haldone (1928) • Early Earth atmosphere (mixture of gases and solar radiation/lightning) • Miller-Urey Experiment Stanley L. Miller - Biography Born: 1930 in Oakland, CA Died: 2007 in San Diego, CA High school nickname: “a chem whiz” BS: UC Berkley - 1951 PhD: University of Chicago – 1954 (advisor: Harold Urey) California Institute of Technology Columbia University UC San Diego (1960-2007) National Academy of Sciences Landmark Paper: (1953) Production of amino acids under possible primitive earth conditions". -

Stanley L. Miller 1930–2007
Stanley L. Miller 1930–2007 A Biographical Memoir by Jeffrey L. Bada and Antonio Lazcano ©2012 National Academy of Sciences. Any opinions expressed in this memoir are those of the authors and do not necessarily reflect the views of the National Academy of Sciences. STANLEY L. MILLER March 7, 1930–May 20, 2007 Elected to the NAS, 1973 Stanley l. Miller, who was considered to be the father of prebiotic chemistry—the synthetic organic chemistry that takes place under natural conditions in geocosmochem- ical environments—passed away on May 20, 2007, at age 77 after a lengthy illness. Stanley was known worldwide for his 1950s demonstration of the prebiotic synthesis of organic compounds, such as amino acids, under simu- lated primitive Earth conditions in the context of the origin of life. On May 15, 1953, while Miller was a graduate student of Harold C. Urey at the University of Chicago, he published a short paper in Science on the synthesis of Stanley Miller Papers, the Mandeville of negavtive in The Register From at California University of the Geisel Library, Special Collection Library at 5. file 163, San Diego; MSS 642, box amino acids under simulated early Earth conditions. This paper and the experiment it described had a tremendous impact and immediately transformed the study of the By Jeffrey L. Bada origin of life into a respectable field of inquiry. and Antonio Lazcano Stanley Lloyd Miller was born in March 7, 1930, in Oakland, California, the second child (the first was his brother, Donald) of Nathan and Edith Miller, descendants of Jewish immigrants from the eastern European countries of Belarus and Latvia. -

MILLER & UREY EXPERIMENT Could Organic Molecules Assemble
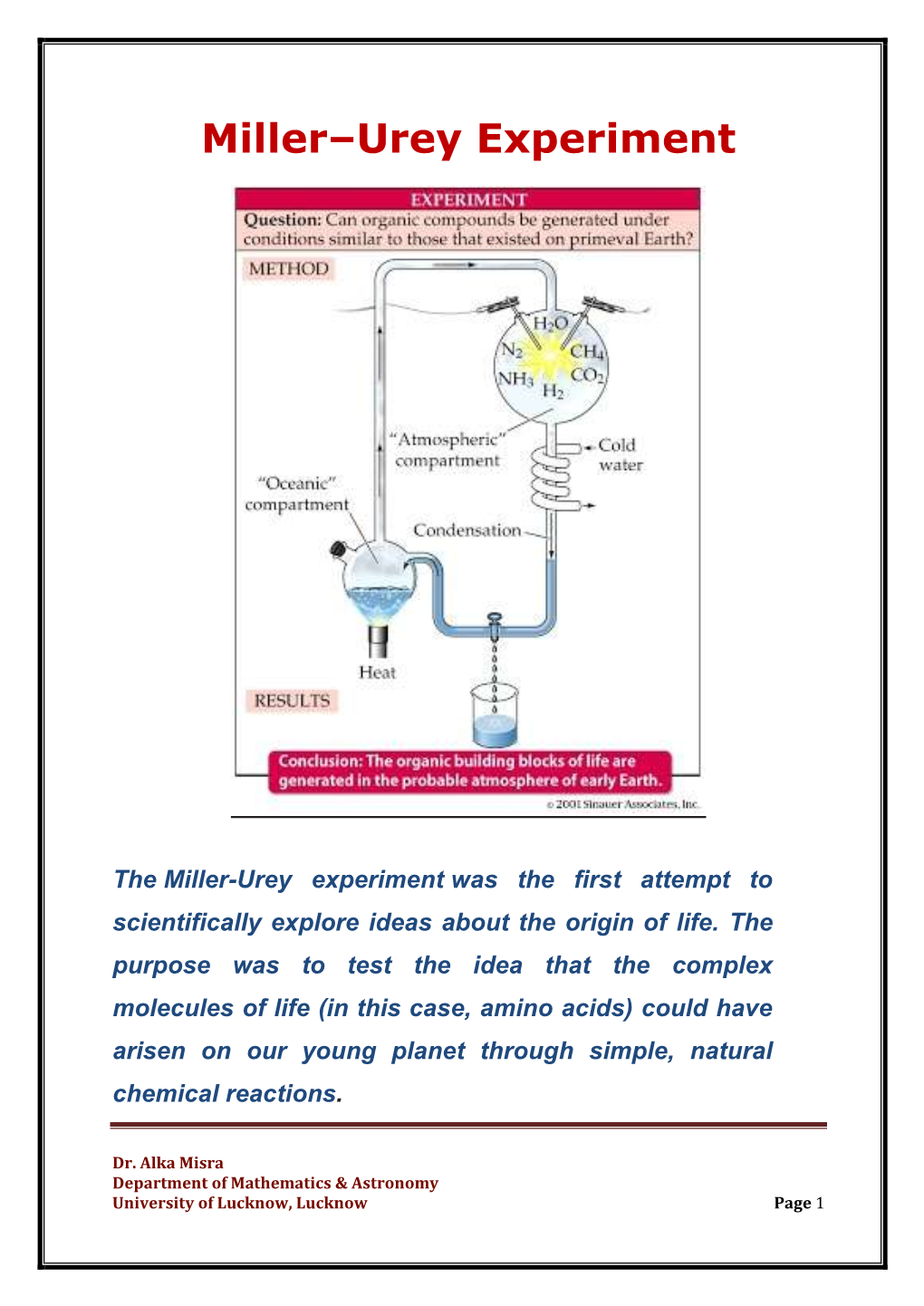
CLASSWORK: ORIGINS OF CELLS PERIOD: NAME: DATE: MILLER & UREY EXPERIMENT Could organic molecules assemble under conditions on early Earth? In 1953, chemists Stanley Miller and Harold Urey tried to answer that question. They filled a sterile flask with water, to simulate the early oceans, and boiled it. To the water vapor, they added methane, ammonia, and hydrogen to simulate the gasses that they thought were in the early atmosphere. Then, as shown in the diagram, they passed the gasses through electrodes to simulate lightning. Next, they passed the gasses through a condensation chamber, where cold water cooled them, causing liquid droplets to form and return to the starting flask. The liquid circulated through the experimental set up for 1 week. The results were spectacular. From these simple molecules, they produced 21 different amino acids – the building blocks of organic proteins. 1. Explain what each part of the experiment shown below represents. (Why did Miller & Urey include each component?) Part of the Experiment: What it Represents/Why it was Included: Heated Water Mix of Gasses (Methane, Ammonia, & Hydrogen) Electric Charge 2. What conclusions can Miller & Urey draw, based on their 1953 experiment? ______________________________________________________________________________________________________________ ______________________________________________________________________________________________________________ 3. We can say that the proteins in this experiment “self-assembled.” Based on your understanding of this experiment, -

Abiogenesis – the Emergence of Life for the Very First Time
Abiogenesis – the emergence of life for the very first time. The question Darwin never addressed; was how life on Earth arose from inorganic matter; the so-called primordial soup. Consider, if life arose once on this planet, that would then mean that all life is related. Ultimately, humans and carrots have a common ancestor; the first proto-cell. Arrogant Worms tell it! Science always proceeds in fits and starts. Pasteur may have disproved abiogensis with his famous swan-neck flask experiments; he still believed that something about life was different. Pasteur believed that all metabolism including fermentation were special reactions that only occur in living organisms; i.e. there something special, maybe even supernatural to life. Pasteur believed that living things (the cells) contained a mysterious ―vital force‖. According to Pasteur, those marvelous macromolecules made by a cell could never be made in a test-tube. Pasteur was unaware of enzymes! Pasteur should have still known better. In 1828, F. Wöhler had reported the first chemical synthesis of a simple organic molecule (urea) from inorganic starting materials (silver cyanate and ammonium chloride). Organic Chemistry has not stopped since! We now think a pre-biotic mix of monomers and polymers accumulated somewhere on our planet. From this mixture rose life for the first and only time, a very very unlikely event – the first proto-cell - explaining why all life shares the same genetic code. How did these molecules first arise and how they were first assembled? Consider the Central Dogma of Genetics: The emergence of life for the first time on this planet constitutes the classic question of what came first; the chicken or the egg?! Did a self-replicating DNA system occur before transcription or translation evolved (the DNA World) or did a self-replicating RNA system first emerge (the RNA world) or did self-replicating protein system first emerge (the Protein World)…or did replication, transcription and translation emerge together all at once. -

The Spark of Life: Where Did Organic Molecules Come From? by Annie Prud’Homme-Généreux, Nicole F
NATIONAL CENTER FOR CASE STUDY TEACHING IN SCIENCE The Spark of Life: Where Did Organic Molecules Come From? by Annie Prud’homme-Généreux, Nicole F. Magill, and Tatiana N. Bliss Life Sciences Quest University Canada Part I – A Flash of Insight I know how to test this! * It was the fall of 1951. Twenty-one-year-old Stanley had recently traveled from his native California to the University of Chicago to pursue a graduate degree in chemistry. At a departmental seminar, his imagination was captured by the presentation from a professor in his department, the Nobel Laureate Harold C. Urey. “In the course of an extended study on the origin of the planets I have come to certain defnite conclusions relative to the early chemical conditions on the Earth and their bearing on the origin of life,” said Urey.** Stan listened intently while Urey continued to explain how the early Earth atmosphere was not as it is today. “One sees that hydrogen (H2) was a prominent constituent of the primitive atmosphere and hence that methane (CH4) was as well. Nitrogen was present as nitrogen gas (N2) at high temperatures but may have been present as ammonia (NH3) or ammonium salt at low temperatures.” Stan was riveted. Urey went on to suggest, as had the Russian biochemist Oparin before him, that organic molecules (compounds containing carbon atoms) might have formed on the early Earth from inorganic gases. T is was provocative because it suggested that the molecules of life (which are organic) could be created by simple chemistry, and it could explain how the building blocks of life were frst created on our primitive lifeless planet. -

Unit 2 – Biochemistry of Life Name ______
Unit 2 – Biochemistry of Life Name __________________________ Chapter 4: Carbon and the Molecular Diversity of Life Overview: Carbon 4.1 Organic chemistry is the study of carbon compounds Organic chemistry focuses on organic compounds containing carbon. o Organic compounds can range from simple molecules, such as CH4, to complex molecules such as proteins, with thousands of atoms. o Most organic compounds contain hydrogen atoms as well as carbon. The overall percentages of the major elements of life (C, H, O, N, S, and P) are quite uniform from one organism to another. Because of carbon’s versatility, these few elements can be combined to build an inexhaustible variety of organic molecules. The science of organic chemistry began with attempts to purify and improve the yield of products obtained from organisms. o Initially, chemists learned to synthesize simple compounds in the laboratory but had no success with more complex compounds. Early organic chemists proposed vitalism, the belief that physical and chemical laws do not apply to living things. o In the early 1800s, the German chemist Friedrich Wöhler and his students synthesized urea. A few years later, Hermann Kolbe, a student of Wöhler’s, made the organic compound acetic acid from inorganic substances prepared directly from pure elements. In 1953, Stanley Miller at the University of Chicago set up a laboratory simulation of possible chemical conditions on the primitive Earth and demonstrated the spontaneous synthesis of organic compounds. o The mixture of gases Miller created probably did not accurately represent the atmosphere of the primitive Earth. o Spontaneous abiotic synthesis of organic compounds, possibly near volcanoes, may have been an early stage in the origin of life on Earth. -

The Miller-Urey Experiment
Icons of Evolution? Why Much of What Jonathan Wells Writes about Evolution is Wrong Alan D. Gishlick, National Center for Science Education THE MILLER-UREY how the early atmosphere was probably differ- EXPERIMENT ent from the atmosphere hypothesized in the original experiment. Wells then claims that the THE EXPERIMENT ITSELF actual atmosphere of the early earth makes the he understanding of the origin of life Miller–Urey type of chemical synthesis was largely speculative until the 1920s, impossible, and asserts that the experiment Twhen Oparin and Haldane, working does not work when an updated atmosphere is independently, proposed a theoretical model used. Therefore, textbooks should either dis- for “chemical evolution.” The Oparin– cuss the experiment as an historically interest- Haldane model suggested that under the ing yet flawed exercise, or not discuss it at all. strongly reducing conditions theorized to have Wells concludes by saying that textbooks been present in the atmosphere of the early should replace their discussions of the Miller– earth (between 4.0 and 3.5 billion years ago), Urey experiment with an “extensive discus- inorganic molecules would spontaneously sion” of all the problems facing research into form organic molecules (simple sugars and the origin of life. amino acids). In 1953, Stanley Miller, along These allegations might seem serious; how- with his graduate advisor Harold Urey, tested ever, Wells’s knowledge of prebiotic chemistry this hypothesis by constructing an apparatus is seriously flawed. First, Wells’s claim that that simulated the Oparin-Haldane “early researchers are ignoring the new atmospheric earth.” When a gas mixture based on predic- data, and that experiments like the Miller– tions of the early atmosphere was heated and Urey experiment fail when the atmospheric given an electrical charge, organic compounds composition reflects current theories, is simply were formed (Miller, 1953; Miller and Urey, false. -

Badacsr 2013
View Article Online View Journal | View Issue This article was published as part of the Prebiotic chemistry themed issue Guest editors Jean-François Lambert, Mariona Sodupe and Piero Ugliengo Please take a look at the issue 16 2012 table of contents to access other reviews in this themed issue Published on 22 January 2013. Downloaded by Rice University 17/10/2015 16:17:08. View Article Online Chem Soc Rev REVIEW ARTICLE New insights into prebiotic chemistry from Cite this: Chem. Soc. Rev., 2013, Stanley Miller’s spark discharge experiments† 42, 2186 Jeffrey L. Bada* 1953 was a banner year for biological chemistry: The double helix structure of DNA was published by Watson and Crick, Sanger’s group announced the first amino acid sequence of a protein (insulin) and the synthesis of key biomolecules using simulated primordial Earth conditions has demonstrated by Miller. Miller’s studies in particular transformed the study of the origin of life into a respectable field of inquiry and established the basis of prebiotic chemistry, a field of research that investigates how the components of life as we know it can be formed in a variety of cosmogeochemical environments. In this review, I cover the continued advances in prebiotic syntheses that Miller’s pioneering work has inspired. The main focus is on recent state-of-the-art analyses carried out on archived samples of Miller’s original experiments, some of which had never before been analyzed, discovered in his laboratory material just before his death in May 2007. One experiment utilized a reducing gas mixture and an apparatus configuration (referred to here as the ‘‘volcanic’’ apparatus) that could represent a water-rich volcanic eruption accompanied by lightning. -

The Origin of Life on Earth by Leslie E. Orgel Growing Evidence
The Origin of Life on Earth by Leslie E. Orgel Growing evidence supports the idea that the emergence of catalytic RNA was a crucial early step. How that RNA came into being remains unknown. LESLIE E. ORGEL is senior fellow and research professor at the Salk Institute for Biological Studies in San Diego, which he joined in 1965. He obtained his Ph.D. in chemistry from the University of Oxford in 1951 and subsequently became a reader in chemistry at the University of Cambridge. While at Cambridge, he contributed to the development of ligand- field theory. The National Aeronautics and Space Administration supports his extensive research on chemistry that may be relevant to the origin of life. Orgel is a fellow of the Royal Society and a member of the National Academy of Sciences. When the earth formed some 4.6 billion years ago, it was a lifeless, inhospitable place. A billion years later it was teeming with organisms resembling blue-green algae. How did they get there? How, in short, did life begin? This long-standing question continues to generate fascinating conjectures and ingenious experiments, many of which center on the possibility that the advent of self- replicating RNA was a critical milestone on the road to life. Before the mid-17th century, most people believed that God had created humankind and other higher organisms and that insects, frogs and other small creatures could arise spontaneously in mud or decaying matter. For the next two centuries, those ideas were subjected to increasingly severe criticism, and in the mid-19th century two important scientific advances set the stage for modern discussions of the origin of life. -

The Origins of Life: a Review of Scientific Inquiry
The Origins of Life: A Review of Scientific Inquiry April 2020 Professor H James Cleaves Earth-Life Science Institute Tokyo Institute of Technology 1 Table of Contents 1. INTRODUCTION .............................................................................................. 3 2. TOP-DOWN AND BOTTOM-UP APPROACHES .................................................... 5 3. HISTORICAL BACKGROUND ............................................................................ 8 4. OVERVIEW OF BIOLOGY ................................................................................ 15 5. OVERVIEW OF SOLAR SYSTEM AND EARTH HISTORY .................................... 18 6. PREBIOTIC CHEMISTRY AND MODELS FOR THE ORIGINS OF LIFE ................ 32 6.1 PREBIOTIC SYNTHESES OF BIOCHEMICALS ............................................................................. 42 Amino Acids ............................................................................................................................................ 42 Lipids and Membrane-Forming Compounds ............................................................................................... 45 Nucleic Acids and Their Components ......................................................................................................... 46 Sugars .................................................................................................................................................... 50 Cofactors and Other Small Molecules ........................................................................................................ -

A Safer Recipe for Primordial Soup 3 February 2014, by Johnny Bontemps
A safer recipe for primordial soup 3 February 2014, by Johnny Bontemps The famous recipe went as follow: Boil some water to mimic evaporation of the early ocean. Add a few gases thought to be present in the early atmosphere. Apply a jolt of electricity to simulate lightning. Let run for a few days—and you're left with a brownish soup of amino acids, the building blocks for everything alive on Earth. But while the success of the Miller-Urey experiment kicked off an entire field of research, Miller had one basic piece of advice for anyone who'd want to try it out: "Don't do it." "Stanley was always afraid it might lead to a disaster," explains Jeffrey Bada, who was a student of Miller in the 1960s. "If you were not careful to let all the atmospheric air out, the setup could explode. So unless they were highly trained, he'd always advise people against repeating the experiment." But now a team including scientists from the Georgia Institute of Technology, NASA (including Dr. Bada), and the Tokyo Institute of Technology have recreated a simpler and safer way of conducting Miller-Urey type experiments. Stanley Miller performs his famous experiment in issue 1 of "Astrobiology: The story of our search for life in the Along with written instructions, the new version was Universe." Credit: NASA Astrobiology/artwork by Aaron published this month in a step-by-step video format Gronstal. in the Journal of Visualized Experiment. "In addition to being simpler and safer, the new configuration provides a better representation of Researchers have published a simpler, safer Earth's early condition," says Eric Parker, a method for conducting Miller-Urey origin of life graduate student at GIT and a lead researcher for experiments—which may still yield new insight the study. -

Primordial Synthesis of Amines and Amino Acids in a 1958 Miller H2 S
Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment Eric T. Parkera,1, Henderson J. Cleavesb, Jason P. Dworkinc, Daniel P. Glavinc, Michael Callahanc, Andrew Aubreyd, Antonio Lazcanoe, and Jeffrey L. Badaa,2 aGeosciences Research Division, Scripps Institution of Oceanography, University of California at San Diego, 8615 Kennel Way, La Jolla, CA 92093; bGeophysical Laboratory, Carnegie Institution of Washington, 5251 Broad Branch Road NW, Washington, DC 20015; cNational Aeronautics and Space Administration Goddard Space Flight Center, Solar System Exploration Division, Greenbelt, MD 20771; dNational Aeronautics and Space Administration Jet Propulsion Laboratory, California Institute of Technology, 4800 Oak Grove Drive, Pasadena, CA 91109; and eFacultad de Ciencias, Universidad Nacional Autónoma de México, Apdo. Postal 70-407 Ciudad Universitaria, 04510 Mexico D.F., Mexico Edited by Gerald F. Joyce, The Scripps Research Institute, La Jolla, CA, and approved February 14, 2011 (received for review December 22, 2010) Archived samples from a previously unreported 1958 Stanley Miller known volcanic apparatus were found to contain a wide variety of electric discharge experiment containing hydrogen sulfide (H2S) amino acids and amines, including ornithine, homoserine, methy- were recently discovered and analyzed using high-performance lamine, and ethylamine, many of which had not been reported liquid chromatography and time-of-flight mass spectrometry. We previously in spark discharge experiments (7). The volcanic report here the detection and quantification of primary amine- apparatus differed from Miller’s classic apparatus in that it uti- containing compounds in the original sample residues, which were lized an aspirator that injected steam into the electric discharge produced via spark discharge using a gaseous mixture of H2S, CH4, chamber, simulating a volcanic eruption (1).