A Production of Amino Acids Under Possible Primitive Earth Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regarding Belief in Primordial Soup to Explain the Origin of Life
Regarding Belief in Primordial Soup to explain the Origin of Life Belief in a primordial soup where life might spontaneously evolve is as wrong as believing the earth is flat, or, as Hoyle and Wickramasinghe (1981) suggest, as wrong as believing that the pre-Copernican idea that the earth is the center of the universe. They state, “It will be no bad thing to abandon this position” (p. 138). The belief in primordial soup goes something like this: “Somehow a brew of appropriate chemicals managed to get together, the organic soup, and somehow the chemicals managed to shuffle themselves into an early primitive life-form. From then on, all appeared to be plain-sailing, natural selection operating on randomly generated mutations would do the rest” (p. 131). Today we know that life does not arise spontaneously. In the 1860s, Louis Pasteur demonstrated that “the concept of spontaneous generation” was incorrect using “carefully designed experiments” (p. 36). Yet many biologists still “believe that life started on Earth through spontaneous processes” (p. 37). “The recourse to an organic soup to cross this biggest hurdle is evidently a blatant recourse to the spontaneous generation theory which Pasteur claimed to have destroyed. Nevertheless, most scientists, even to this day, have been satisfied to accept it” (p. 37). What scientific tests did Louis Pasteur conduct about spontaneous generation of life? How did he set up his experiment? What were his steps? What was his result? _________________________________________________________________ _________________________________________________________________ -

The Spontaneous Generation Controversy (340 BCE–1870 CE)
270 4. Abstraction and Unification ∗ ∗ ∗ “O`uen ˆetes-vous? Que faites-vous? Il faut travailler” (on his death-bed, to his devoted pupils, watching over him). The Spontaneous Generation Controversy (340 BCE–1870 CE) “Omne vivium ex Vivo.” (Latin proverb) Although the theory of spontaneous generation (abiogenesis) can be traced back at least to the Ionian school (600 B.C.), it was Aristotle (384-322 B.C.) who presented the most complete arguments for and the clearest statement of this theory. In his “On the Origin of Animals”, Aristotle states not only that animals originate from other similar animals, but also that living things do arise and always have arisen from lifeless matter. Aristotle’s theory of sponta- neous generation was adopted by the Romans and Neo-Platonic philosophers and, through them, by the early fathers of the Christian Church. With only minor modifications, these philosophers’ ideas on the origin of life, supported by the full force of Christian dogma, dominated the mind of mankind for more that 2000 years. According to this theory, a great variety of organisms could arise from lifeless matter. For example, worms, fireflies, and other insects arose from morning dew or from decaying slime and manure, and earthworms originated from soil, rainwater, and humus. Even higher forms of life could originate spontaneously according to Aristotle. Eels and other kinds of fish came from the wet ooze, sand, slime, and rotting seaweed; frogs and salamanders came from slime. 1846 CE 271 Rather than examining the claims of spontaneous generation more closely, Aristotle’s followers concerned themselves with the production of even more remarkable recipes. -

Aristotle on Chance and Spontaneous Generation. a Discussion Note
FILOZOFIA ___________________________________________________________________________Roč. 68, 2013, č. 2 ARISTOTLE ON CHANCE AND SPONTANEOUS GENERATION. A DISCUSSION NOTE CHRISTOS PANAYIDES, University of Nicosia, Cyprus PANAYIDES, CH.: Aristotle on Chance and Spontaneous Generation. A Discussion Note FILOZOFIA 68, 2013, No 2, p. 114 In Physics II. 4-6 Aristotle deals with the technical concept of chance (τ ατ µατον). Here a number of specific characteristics are ascribed to the chance happenings. At the same time, in his biological works Aristotle presents his notorious theory of ‘spontaneous generation’ (ατ µατος γ9νεσις). Most scholars assume that this theory ought to be in line with the doctrine of chance, as this is presented in his Physics. It is clear, however, that spontaneous generation lacks (at least some of) the features a chance happening ought to have. For instance, spontaneity is not unusual. My pur- pose here is to address the exegetical problem at hand, in particular to sketch out an argument according to which the discrepancy between Aristotle’s doctrine of chance and his theory of spontaneous generation is merely an apparent one. Keywords: Aristotle – Chance (being) for the sake of something – Generation of ani- mals – Incidental causation J. Lennox – Luck – Per se causation – Physics I In Physics (Phys.) II. 4-6 Aristotle deals with the technical concepts of ‘luck’ (Dτχη) and ‘chance’ (τ ατ µατον). In the opening paragraph of Phys. II. 4 (195b31- 36) he states that the ensuing discussion, in the next couple of chapters, is intended to do three things: (a) determine what luck and chance are, (b) resolve how, if at all, these two fit into the scheme of the four causes and (c) specify how luck and chance are related to 1 each other. -

Stanley L. Miller 1930–2007
Stanley L. Miller 1930–2007 A Biographical Memoir by Jeffrey L. Bada and Antonio Lazcano ©2012 National Academy of Sciences. Any opinions expressed in this memoir are those of the authors and do not necessarily reflect the views of the National Academy of Sciences. STANLEY L. MILLER March 7, 1930–May 20, 2007 Elected to the NAS, 1973 Stanley l. Miller, who was considered to be the father of prebiotic chemistry—the synthetic organic chemistry that takes place under natural conditions in geocosmochem- ical environments—passed away on May 20, 2007, at age 77 after a lengthy illness. Stanley was known worldwide for his 1950s demonstration of the prebiotic synthesis of organic compounds, such as amino acids, under simu- lated primitive Earth conditions in the context of the origin of life. On May 15, 1953, while Miller was a graduate student of Harold C. Urey at the University of Chicago, he published a short paper in Science on the synthesis of Stanley Miller Papers, the Mandeville of negavtive in The Register From at California University of the Geisel Library, Special Collection Library at 5. file 163, San Diego; MSS 642, box amino acids under simulated early Earth conditions. This paper and the experiment it described had a tremendous impact and immediately transformed the study of the By Jeffrey L. Bada origin of life into a respectable field of inquiry. and Antonio Lazcano Stanley Lloyd Miller was born in March 7, 1930, in Oakland, California, the second child (the first was his brother, Donald) of Nathan and Edith Miller, descendants of Jewish immigrants from the eastern European countries of Belarus and Latvia. -

Spontaneous Generation & Origin of Life Concepts from Antiquity to The
SIMB News News magazine of the Society for Industrial Microbiology and Biotechnology April/May/June 2019 V.69 N.2 • www.simbhq.org Spontaneous Generation & Origin of Life Concepts from Antiquity to the Present :ŽƵƌŶĂůŽĨ/ŶĚƵƐƚƌŝĂůDŝĐƌŽďŝŽůŽŐLJΘŝŽƚĞĐŚŶŽůŽŐLJ Impact Factor 3.103 The Journal of Industrial Microbiology and Biotechnology is an international journal which publishes papers in metabolic engineering & synthetic biology; biocatalysis; fermentation & cell culture; natural products discovery & biosynthesis; bioenergy/biofuels/biochemicals; environmental microbiology; biotechnology methods; applied genomics & systems biotechnology; and food biotechnology & probiotics Editor-in-Chief Ramon Gonzalez, University of South Florida, Tampa FL, USA Editors Special Issue ^LJŶƚŚĞƚŝĐŝŽůŽŐLJ; July 2018 S. Bagley, Michigan Tech, Houghton, MI, USA R. H. Baltz, CognoGen Biotech. Consult., Sarasota, FL, USA Impact Factor 3.500 T. W. Jeffries, University of Wisconsin, Madison, WI, USA 3.000 T. D. Leathers, USDA ARS, Peoria, IL, USA 2.500 M. J. López López, University of Almeria, Almeria, Spain C. D. Maranas, Pennsylvania State Univ., Univ. Park, PA, USA 2.000 2.505 2.439 2.745 2.810 3.103 S. Park, UNIST, Ulsan, Korea 1.500 J. L. Revuelta, University of Salamanca, Salamanca, Spain 1.000 B. Shen, Scripps Research Institute, Jupiter, FL, USA 500 D. K. Solaiman, USDA ARS, Wyndmoor, PA, USA Y. Tang, University of California, Los Angeles, CA, USA E. J. Vandamme, Ghent University, Ghent, Belgium H. Zhao, University of Illinois, Urbana, IL, USA 10 Most Cited Articles Published in 2016 (Data from Web of Science: October 15, 2018) Senior Author(s) Title Citations L. Katz, R. Baltz Natural product discovery: past, present, and future 103 Genetic manipulation of secondary metabolite biosynthesis for improved production in Streptomyces and R. -

MILLER & UREY EXPERIMENT Could Organic Molecules Assemble
CLASSWORK: ORIGINS OF CELLS PERIOD: NAME: DATE: MILLER & UREY EXPERIMENT Could organic molecules assemble under conditions on early Earth? In 1953, chemists Stanley Miller and Harold Urey tried to answer that question. They filled a sterile flask with water, to simulate the early oceans, and boiled it. To the water vapor, they added methane, ammonia, and hydrogen to simulate the gasses that they thought were in the early atmosphere. Then, as shown in the diagram, they passed the gasses through electrodes to simulate lightning. Next, they passed the gasses through a condensation chamber, where cold water cooled them, causing liquid droplets to form and return to the starting flask. The liquid circulated through the experimental set up for 1 week. The results were spectacular. From these simple molecules, they produced 21 different amino acids – the building blocks of organic proteins. 1. Explain what each part of the experiment shown below represents. (Why did Miller & Urey include each component?) Part of the Experiment: What it Represents/Why it was Included: Heated Water Mix of Gasses (Methane, Ammonia, & Hydrogen) Electric Charge 2. What conclusions can Miller & Urey draw, based on their 1953 experiment? ______________________________________________________________________________________________________________ ______________________________________________________________________________________________________________ 3. We can say that the proteins in this experiment “self-assembled.” Based on your understanding of this experiment, -

History of Microbiology: Spontaneous Generation Theory
HISTORY OF MICROBIOLOGY: SPONTANEOUS GENERATION THEORY Microbiology often has been defined as the study of organisms and agents too small to be seen clearly by the unaided eye—that is, the study of microorganisms. Because objects less than about one millimeter in diameter cannot be seen clearly and must be examined with a microscope, microbiology is concerned primarily with organisms and agents this small and smaller. Microbial World Microorganisms are everywhere. Almost every natural surface is colonized by microbes (including our skin). Some microorganisms can live quite happily in boiling hot springs, whereas others form complex microbial communities in frozen sea ice. Most microorganisms are harmless to humans. You swallow millions of microbes every day with no ill effects. In fact, we are dependent on microbes to help us digest our food and to protect our bodies from pathogens. Microbes also keep the biosphere running by carrying out essential functions such as decomposition of dead animals and plants. Microbes are the dominant form of life on planet Earth. More than half the biomass on Earth consists of microorganisms, whereas animals constitute only 15% of the mass of living organisms on Earth. This Microbiology course deals with • How and where they live • Their structure • How they derive food and energy • Functions of soil micro flora • Role in nutrient transformation • Relation with plant • Importance in Industries The microorganisms can be divided into two distinct groups based on the nucleus structure: Prokaryotes – The organism lacking true nucleus (membrane enclosed chromosome and nucleolus) and other organelles like mitochondria, golgi body, entoplasmic reticulum etc. are referred as Prokaryotes. -
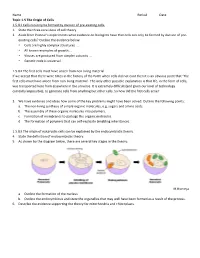
Topic 1.5 RG
Name ________________________________________________________________ Period ______ Date ____________ Topic 1.5 The Origin of Cells 1.5.U1 Cells can only be formed by division of pre-existing cells. 1. State the three core ideas of cell theory. 2. Aside from Pasteur’s experiments what evidence do Biologists have that cells can only be formed by division of pre- existing cells? Outline the evidence below: • Cells are highly complex structures ... • All known examples of growth ... • Viruses are produced from simpler subunits ... • Genetic code is universal ... 1.5.U2 The first cells must have arisen from non-living material. If we accept that there were times in the history of the Earth when cells did not exist then it is an obvious point that ‘The first cells must have arisen from non-living material’. The only other possible explanation is that life, in the form of cells, was transported here from elsewhere in the universe. It is extremely difficult (and given our level of technology currently impossible), to generate cells from anything but other cells. So how did the first cells arise? 3. We have evidence and ideas how some of the key problems might have been solved. Outline the following points: a. The non-living synthesis of simple organic molecules, e.g. sugars and amino acids. b. The assembly of these organic molecules into polymers. c. Formation of membranes to package the organic molecules. d. The formation of polymers that can self-replicate (enabling inheritance). 1.5.U3 The origin of eukaryotic cells can be explained by the endosymbiotic theory. 4. State the definition of endosymbiotic theory. -

Spontaneous Generation Vs. Biogenesis SCIENTIFIC Classic Experiments by Redi, Spallinzani, and Pasteur BIO FAX!
Spontaneous Generation vs. Biogenesis SCIENTIFIC Classic Experiments by Redi, Spallinzani, and Pasteur BIO FAX! Introduction Where do living things come from? Do they arise from non-living materials, or can they only come from pre-existing living things? Recreate three classic experiments that helped to disprove the theory of spontaneous generation. Concepts •Biogenesis • Spontaneous generation • Sterilization Materials (for each demonstration or group) Bleach solution (sodium hypochlorite), 10%, 400 mL Marker or wax pencil Chicken, beef or liver, 1⁄3 × 1⁄3 cubed, 2 Plastic tubing, 1⁄8 × 1 Nutrient broth, powder, 2 g Plastic tubing, 1⁄8 × 2 Water, distilled or deionized Plastic tubing, 1⁄8 × 3 Autoclave or pressure cooker Plugs, foam, 21–26 mm, 10 Beaker, 500-mL Stirring rod Cork borer, 1⁄8 Tape Gauze, 1 × 1 Test tube rack Gloves, latex, or other disposable type Test tubes, 25 × 150 mm, 9 Graduated cylinder, 25 mL Tongs Hot plate Safety Precautions Be sure to follow directions carefully when using an autoclave or pressure cooker. Sodium hypochlorite (bleach) causes skin burns and is toxic by ingestion. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Follow all laboratory safety guidelines and wash hands thoroughly with soap and water before leaving the laboratory. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Procedure Part A. Francisco Redi’s 1668 experiment Hypothesis: Living matter always arises from pre-existing living matter. 1. Label two test tubes “A” and “B.” Place a piece of meat in each test tube. 2. Allow test tube “A” to remain open. -

Chapter 14 History of Life
Chapter 14 History of Life Section 1 Biogenesis Section 2 Earth’s History Section 3 The First Life-Forms 14-1 Biogenesis Objectives: Compare the principle of biogenesis with the idea of spontaneous generation. Summarize the results of experiments by Redi and by Spallanzani that tested the hypothesis of spontaneous generation. Describe how Pasteur’s experiment disproved the hypothesis of spontaneous generation. Redi's Experiments Redi, Francesco - 1629-1690 Before the 1600s, it was generally thought that organisms could arise from nonliving material by spontaneous generation. Pilgrims landed on Plymouth Rock in 1620 Redi showed in 1668 that rotting meat kept away from flies would not produce new flies. Maggots appeared only on meat that had been exposed to flies. Lazzaro Spallanzani's Experiments Spallanzani showed in the 1700s that microorganisms would not grow in broth when its container was heated and then sealed. He inferred that microorganisms do not arise spontaneously but, rather, are carried in the air. Critics claimed "vital force" is essential and Spallanzani destroyed it! Pasteur's Experiment Louis Pasteur (1822-1895) in the 1800s used a variation of Spallanzani’s design to prove that microorganisms are carried in the air and do not arise by spontaneous generation. 14-2 Earth's History Outline the modern scientific understanding of the formation of Earth. Summarize the concept of half-life. Describe the production of organic compounds in the Miller-Urey apparatus. Summarize the possible importance of cell-like structures produced in the laboratory. Formation of Earth 4.6 to Be exact Earth’s Age Scientists think that Earth formed more than 4 billion years ago by the gravitational accumulation of dust and debris moving through space. -

Abiogenesis – the Emergence of Life for the Very First Time
Abiogenesis – the emergence of life for the very first time. The question Darwin never addressed; was how life on Earth arose from inorganic matter; the so-called primordial soup. Consider, if life arose once on this planet, that would then mean that all life is related. Ultimately, humans and carrots have a common ancestor; the first proto-cell. Arrogant Worms tell it! Science always proceeds in fits and starts. Pasteur may have disproved abiogensis with his famous swan-neck flask experiments; he still believed that something about life was different. Pasteur believed that all metabolism including fermentation were special reactions that only occur in living organisms; i.e. there something special, maybe even supernatural to life. Pasteur believed that living things (the cells) contained a mysterious ―vital force‖. According to Pasteur, those marvelous macromolecules made by a cell could never be made in a test-tube. Pasteur was unaware of enzymes! Pasteur should have still known better. In 1828, F. Wöhler had reported the first chemical synthesis of a simple organic molecule (urea) from inorganic starting materials (silver cyanate and ammonium chloride). Organic Chemistry has not stopped since! We now think a pre-biotic mix of monomers and polymers accumulated somewhere on our planet. From this mixture rose life for the first and only time, a very very unlikely event – the first proto-cell - explaining why all life shares the same genetic code. How did these molecules first arise and how they were first assembled? Consider the Central Dogma of Genetics: The emergence of life for the first time on this planet constitutes the classic question of what came first; the chicken or the egg?! Did a self-replicating DNA system occur before transcription or translation evolved (the DNA World) or did a self-replicating RNA system first emerge (the RNA world) or did self-replicating protein system first emerge (the Protein World)…or did replication, transcription and translation emerge together all at once. -

The Spark of Life: Where Did Organic Molecules Come From? by Annie Prud’Homme-Généreux, Nicole F
NATIONAL CENTER FOR CASE STUDY TEACHING IN SCIENCE The Spark of Life: Where Did Organic Molecules Come From? by Annie Prud’homme-Généreux, Nicole F. Magill, and Tatiana N. Bliss Life Sciences Quest University Canada Part I – A Flash of Insight I know how to test this! * It was the fall of 1951. Twenty-one-year-old Stanley had recently traveled from his native California to the University of Chicago to pursue a graduate degree in chemistry. At a departmental seminar, his imagination was captured by the presentation from a professor in his department, the Nobel Laureate Harold C. Urey. “In the course of an extended study on the origin of the planets I have come to certain defnite conclusions relative to the early chemical conditions on the Earth and their bearing on the origin of life,” said Urey.** Stan listened intently while Urey continued to explain how the early Earth atmosphere was not as it is today. “One sees that hydrogen (H2) was a prominent constituent of the primitive atmosphere and hence that methane (CH4) was as well. Nitrogen was present as nitrogen gas (N2) at high temperatures but may have been present as ammonia (NH3) or ammonium salt at low temperatures.” Stan was riveted. Urey went on to suggest, as had the Russian biochemist Oparin before him, that organic molecules (compounds containing carbon atoms) might have formed on the early Earth from inorganic gases. T is was provocative because it suggested that the molecules of life (which are organic) could be created by simple chemistry, and it could explain how the building blocks of life were frst created on our primitive lifeless planet.