Postgenomic Global Analysis of Translational Control Induced by Oncogenic Signaling
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Translational Control in Cancer Etiology
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Translational Control in Cancer Etiology Davide Ruggero Helen Diller Cancer Center, School of Medicine, University of California, San Francisco, California 94158 Correspondence: [email protected] The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as “ribosomopathies” associated with in- creased cancer susceptibility. These discoveries have illuminated the importance of dereg- ulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by in- creasing overall protein synthesis and by modulating specific mRNA networks. These trans- lational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer for- mation and offers significant promise for innovative cancer therapies. Current research, -

Structural Characterization of the Human Eukaryotic Initiation Factor 3 Protein Complex by Mass Spectrometry*□S
Supplemental Material can be found at: http://www.mcponline.org/cgi/content/full/M600399-MCP200 /DC1 Research Structural Characterization of the Human Eukaryotic Initiation Factor 3 Protein Complex by Mass Spectrometry*□S Eugen Damoc‡, Christopher S. Fraser§, Min Zhou¶, Hortense Videler¶, Greg L. Mayeurʈ, John W. B. Hersheyʈ, Jennifer A. Doudna§, Carol V. Robinson¶**, and Julie A. Leary‡ ‡‡ Protein synthesis in mammalian cells requires initiation The initiation phase of eukaryotic protein synthesis involves factor eIF3, an ϳ800-kDa protein complex that plays a formation of an 80 S ribosomal complex containing the initi- Downloaded from central role in binding of initiator methionyl-tRNA and ator methionyl-tRNAi bound to the initiation codon in the mRNA to the 40 S ribosomal subunit to form the 48 S mRNA. This is a multistep process promoted by proteins initiation complex. The eIF3 complex also prevents pre- called eukaryotic initiation factors (eIFs).1 Currently at least 12 mature association of the 40 and 60 S ribosomal subunits eIFs, composed of at least 29 distinct subunits, have been and interacts with other initiation factors involved in start identified (1). Mammalian eIF3, the largest initiation factor, is a codon selection. The molecular mechanisms by which multisubunit complex with an apparent molecular mass of www.mcponline.org eIF3 exerts these functions are poorly understood. Since ϳ800 kDa. This protein complex plays an essential role in its initial characterization in the 1970s, the exact size, translation by binding directly to the 40 S ribosomal subunit composition, and post-translational modifications of and promoting formation of the 43 S preinitiation complex ⅐ ⅐ mammalian eIF3 have not been rigorously determined. -
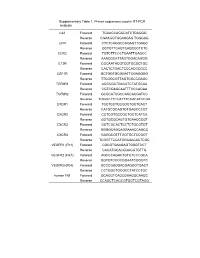
Supplementary Table 1. Primer Sequences Used in RT-PCR Analysis
Supplementary Table 1. Primer sequences used in RT-PCR analysis L32 Forward TGAAGCAGGCATCTGAGGG Reverse CGAAGGTGGAAGAG TGGGAG LIFR Forward CTCTCAGGCCAGAGTTGAGC Reverse GCTGTTCAGTCAGCCCTCTC CCR2 Forward TGTCTTCCCTGAATTGAGCC Reverse AAACGCATTAGTGGACAGGG IL12R Forward CGCAATACGTCGTGCGCTGC Reverse CACTCTGACTCCCACGCGCC CSF1R Forward GCTGGTGCGGATTCGAGGGG Reverse TTCGGCGTTAGTGGCCGAGC TGFbR1 Forward ACGCGCTGACATCTATGCAA Reverse CGTCGAGCAATTTCCCAGAA TGFbR2 Forward GCGCATCGCCAGCACGATCC Reverse TGGGCTTCCATTTCCACATCCGA CXCR1 Forward TCCTCCTGCCGCTGCTCACT Reverse CATGCGCAGTGTGAGCCCGT CXCR2 Forward CCTCGTGCCGCTGCTCATCA Reverse GGTGCGCAGTGTGAACCCGT CXCR3 Forward GGTCGCACTGCTCTGCGTGT Reverse GGGGCAGCAGGAAACCAGCC CXCR4 Forward GAGGCGTTTGGTGCTCCGGT Reverse TCGGTTCCATGGCAACACTCGC VEGFR1 (Flt1) Forward CGCGTGAAGAGTGGGTCCT Reverse CACATGCACGGAGGTGTTG VEGFR2 (Flk1) Forward AGCCCAGACTGTGTCCCGCA Reverse GGTGTCCGCGGAATCGGGTC VEGFR3 (Flt4) Forward GCCCGAGGACGAGGGTGACT Reverse CCTGGCTGCGCCTATCCTGC human Flt1 Forward GCACGTCAGCGAAGGCAAGC Reverse CCAGCTCAGCGTGGTCGTAGG Supplementary Table 2. List of putative miR-200 target genes amplified in human cancers amplified in cancers (Tumorscape)a Gene Symbol Title various cancers NSCLC PCT Errfi1 ERBB receptor feedback inhibitor 1 - yes 0.94 D11Bwg0517e DNA segment, Chr 11, Brigham & Women's Genetics 0517 expressed - yes 0.91 Pvrl4 poliovirus receptor-related 4 yes - 0.91 Mecp2 methyl CpG binding protein 2 yes yes 0.9 Jun Jun oncogene yes - 0.88 Prdm16 PR domain containing 16 - yes 0.88 Flt1 FMS-like tyrosine kinase 1 yes -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

4-6 Weeks Old Female C57BL/6 Mice Obtained from Jackson Labs Were Used for Cell Isolation
Methods Mice: 4-6 weeks old female C57BL/6 mice obtained from Jackson labs were used for cell isolation. Female Foxp3-IRES-GFP reporter mice (1), backcrossed to B6/C57 background for 10 generations, were used for the isolation of naïve CD4 and naïve CD8 cells for the RNAseq experiments. The mice were housed in pathogen-free animal facility in the La Jolla Institute for Allergy and Immunology and were used according to protocols approved by the Institutional Animal Care and use Committee. Preparation of cells: Subsets of thymocytes were isolated by cell sorting as previously described (2), after cell surface staining using CD4 (GK1.5), CD8 (53-6.7), CD3ε (145- 2C11), CD24 (M1/69) (all from Biolegend). DP cells: CD4+CD8 int/hi; CD4 SP cells: CD4CD3 hi, CD24 int/lo; CD8 SP cells: CD8 int/hi CD4 CD3 hi, CD24 int/lo (Fig S2). Peripheral subsets were isolated after pooling spleen and lymph nodes. T cells were enriched by negative isolation using Dynabeads (Dynabeads untouched mouse T cells, 11413D, Invitrogen). After surface staining for CD4 (GK1.5), CD8 (53-6.7), CD62L (MEL-14), CD25 (PC61) and CD44 (IM7), naïve CD4+CD62L hiCD25-CD44lo and naïve CD8+CD62L hiCD25-CD44lo were obtained by sorting (BD FACS Aria). Additionally, for the RNAseq experiments, CD4 and CD8 naïve cells were isolated by sorting T cells from the Foxp3- IRES-GFP mice: CD4+CD62LhiCD25–CD44lo GFP(FOXP3)– and CD8+CD62LhiCD25– CD44lo GFP(FOXP3)– (antibodies were from Biolegend). In some cases, naïve CD4 cells were cultured in vitro under Th1 or Th2 polarizing conditions (3, 4). -

Eif2b Is a Decameric Guanine Nucleotide Exchange Factor with a G2e2 Tetrameric Core
ARTICLE Received 19 Feb 2014 | Accepted 15 Apr 2014 | Published 23 May 2014 DOI: 10.1038/ncomms4902 OPEN eIF2B is a decameric guanine nucleotide exchange factor with a g2e2 tetrameric core Yuliya Gordiyenko1,2,*, Carla Schmidt1,*, Martin D. Jennings3, Dijana Matak-Vinkovic4, Graham D. Pavitt3 & Carol V. Robinson1 eIF2B facilitates and controls protein synthesis in eukaryotes by mediating guanine nucleotide exchange on its partner eIF2. We combined mass spectrometry (MS) with chemical cross- linking, surface accessibility measurements and homology modelling to define subunit stoichiometry and interactions within eIF2B and eIF2. Although it is generally accepted that eIF2B is a pentamer of five non-identical subunits (a–e), here we show that eIF2B is a decamer. MS and cross-linking of eIF2B complexes allows us to propose a model for the subunit arrangements within eIF2B where the subunit assembly occurs through catalytic g- and e-subunits, with regulatory subunits arranged in asymmetric trimers associated with the core. Cross-links between eIF2 and eIF2B allow modelling of interactions that contribute to nucleotide exchange and its control by eIF2 phosphorylation. Finally, we identify that GTP binds to eIF2Bg, prompting us to propose a multi-step mechanism for nucleotide exchange. 1 Department of Chemistry, Physical and Theoretical Chemistry Laboratory, University of Oxford, South Parks Road, Oxford OX1 3QZ, UK. 2 MRC Laboratory of Molecular Biology, University of Cambridge, Francis Crick Avenue, Cambridge CB2 0QH, UK. 3 Faculty of Life Sciences, Michael Smith Building, University of Manchester, Oxford Road, Manchester M13 9PT, UK. 4 Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK. -

The Yeast Eif3 Subunits TIF32/A, NIP1/C, and Eif5 Make Critical Connections with the 40Sribosome in Vivo
Downloaded from genesdev.cshlp.org on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press The yeast eIF3 subunits TIF32/a, NIP1/c, and eIF5 make critical connections with the 40Sribosome in vivo Leoš Valášek, Amy A. Mathew, Byung-Sik Shin, Klaus H. Nielsen, Béla Szamecz, and Alan G. Hinnebusch1 Laboratory of Gene Regulation and Development, National Institute of Child Health and Human Development, Bethesda, Maryland 20892, USA Initiation factor 3 (eIF3) forms a multifactor complex (MFC) with eIF1, eIF2, and eIF5 that stimulates Met Met-tRNAi binding to 40Sribosomes and promotes scanning or AUG recognition. We have previously characterized MFC subcomplexes produced in vivo from affinity-tagged eIF3 subunits lacking discrete binding domains for other MFC components. Here we asked whether these subcomplexes can bind to 40Sribosomes in vivo. We found that the N- and C-terminal domains of NIP1/eIF3c, the N- and C-terminal domains of TIF32/eIF3a, and eIF5 have critical functions in 40Sbinding, with eIF5 an d the TIF32-CTD performing redundant functions. The TIF32-CTD interacted in vitro with helices 16–18 of domain I in 18SrRNA, and the TIF32-NTD and NIP1 interacted with 40Sprotein RPS0A.These results sugge st that eIF3 binds to the solvent side of the 40Ssubunit in a way that provides access to the interface side fo r the two eIF3 segments Met (NIP1-NTD and TIF32-CTD) that interact with eIF1, eIF5, and the eIF2/GTP/Met-tRNAi ternary complex. [Keywords: Eukaryotic translation initiation factor (eIF); multifactor complex (MFC); translational control; protein synthesis; 40S ribosome binding; TIF32/NIP1] Supplemental material is available at http://www.genesdev.org. -

Knockdown of EIF5A2 Inhibits the Malignant Potential of Non-Small Cell Lung Cancer Cells
ONCOLOGY LETTERS 15: 4541-4549, 2018 Knockdown of EIF5A2 inhibits the malignant potential of non-small cell lung cancer cells CHENG CHEN1*, BOJIA ZHANG2*, SHANSHAN WU3, YONGXIANG SONG1 and JIAN LI1 1Department of Thoracic Surgery, Affiliated Hospital of Zunyi Medical College, Zunyi, Guizhou 563000; 2Department of Infectious Disease, Shanghai 10th People's Hospital, Tongji University School of Medicine, Shanghai 200072; 3Department of Nursing, Affiliated Hospital of Zunyi Medical College, Zunyi, Guizhou 563000, P.R. China Received March 16, 2016; Accepted February 23, 2017 DOI: 10.3892/ol.2018.7832 Abstract. Eukaryotic translation initiation factor 5A2 through these proteins. EIF5A2 may therefore serve as a novel (EIF5A2) has been demonstrated to be upregulated in therapeutic target for the treatment of NSCLC. numerous types of human cancer and is associated with cancer progression. However, the expression and role of Introduction EIF5A2 in non-small cell lung cancer (NSCLC) remains unclear. In the present study, the role of EIF5A2 in NSCLC Non-small cell lung cancer (NSCLC) accounts for 85% of was investigated, in addition to the underlying molecular pulmonary malignancies (1) and is one of the leading causes of mechanisms by which EIF5A2 acts. Relative EIF5A2 expres- cancer-associated mortality worldwide. Recurrent and meta- sion levels were determined in NSCLC cells and compared static disease is the main cause of mortality, and the 5-year with levels in non-cancerous lung tissues. Short interfering survival rate for lung cancer is 15% (2). Therefore, in order to (si)RNA targeted against EIF5A2 was used to knock down improve the prognosis for patients with NSCLC, it is impor- EIF5A2 levels in NSCLC cells. -

EIF5A2 Controls Ovarian Tumor Growth and Metastasis by Promoting Epithelial to Mesenchymal Transition Via the Title Tgfβ Pathway
EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the Title TGFβ pathway Zhao, Guannan; Zhang, Wenjing; Dong, Peixin; Watari, Hidemichi; Guo, Yuqi; Pfeffer, Lawrence M; Tigyi, Gabor; Author(s) Yue, Junming Cell & Bioscience, 11, 70 Citation https://doi.org/10.1186/s13578-021-00578-5 Issue Date 2021-04-07 Doc URL http://hdl.handle.net/2115/80967 Rights(URL) https://creativecommons.org/licenses/by/4.0/ Type article File Information Yue EIF5A2 paper.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Zhao et al. Cell Biosci (2021) 11:70 https://doi.org/10.1186/s13578-021-00578-5 Cell & Bioscience RESEARCH Open Access EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the TGFβ pathway Guannan Zhao1,2, Wenjing Zhang3, Peixin Dong4, Hidemichi Watari4, Yuqi Guo5*, Lawrence M. Pfefer1,2, Gabor Tigyi6 and Junming Yue1,2* Abstract Background: Epithelial to mesenchymal transition (EMT) contributes to tumor metastasis and chemoresistance. Eukaryotic initiation factor 5A2 (EIF5A2) is highly expressed in a variety of human cancers but rarely expressed in normal tissues. While EIF5A2 has oncogenic activity in several cancers and contributes to tumor metastasis, its role in ovarian cancer is unknown. In this study, we investigate whether EIF5A2 contributes to ovarian tumor metastasis by promoting EMT. Methods: To investigate the role of EIF5A2, we knocked out (KO) EIF5A2 using lentiviral CRISPR/Cas9 nickase in high invasive SKOV3 and OVCAR8 cells and overexpressed EIF5A2 in low invasive OVCAR3 cells using lentiviral vector. Cell proliferation, migration and invasion was examined in vitro ovarian cancer cells and tumor metastasis was evaluated in vivo using orthotopic ovarian cancer mouse models. -

Cofactor Dependent Conformational Switching of Gtpases
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector 1704 Biophysical Journal Volume 95 August 2008 1704–1715 Cofactor Dependent Conformational Switching of GTPases Vasili Hauryliuk,*y Sebastian Hansson,z and Ma˚ns Ehrenberg* *Department of Cell and Molecular Biology, Molecular Biology Program, Uppsala University, Uppsala, Sweden; yEngelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia; and zLaboratoire d’Enzymologie et Biochimie Structurales, CNRS, Avenue de la Terrasse, 91198 Gif-sur-Yvette, France ABSTRACT This theoretical work covers structural and biochemical aspects of nucleotide binding and GDP/GTP exchange of GTP hydrolases belonging to the family of small GTPases. Current models of GDP/GTP exchange regulation are often based on two specific assumptions. The first is that the conformation of a GTPase is switched by the exchange of the bound nucleotide from GDP to GTP or vice versa. The second is that GDP/GTP exchange is regulated by a guanine nucleotide exchange factor, which stabilizes a GTPase conformation with low nucleotide affinity. Since, however, recent biochemical and structural data seem to contradict this view, we present a generalized scheme for GTPase action. This novel ansatz accounts for those important cases when conformational switching in addition to guanine nucleotide exchange requires the presence of cofactors, and gives a more nuanced picture of how the nucleotide exchange is regulated. The scheme is also used to discuss some problems of interpretation that may arise when guanine nucleotide exchange mechanisms are inferred from experiments with analogs of GTP, like GDPNP, GDPCP, and GDP g S. -

Dysregulation of Rho Gtpases in the Apix/Arhgef6 Mouse Model of X
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2011 Dysregulation of Rho GTPases in the Pix/Arhgef6 mouse model of X-linked intellectual disability is paralleled by impaired structural and synaptic plasticity and cognitive deficits Ramakers, G J A ; Wolfer, D ; Rosenberger, G ; Kuchenbecker, K ; Kreienkamp, H J ; Prange-Kiel, J ; Rune, G ; Richter, K ; Langnaese, K ; Masneuf, S ; Bösl, M R ; Fischer, K D ; Krugers, H J ; Lipp, H P ; van Galen, E ; Kutsche, K Abstract: Mutations in the ARHGEF6 gene, encoding the guanine nucleotide exchange factor PIX/Cool- 2 for the Rho GTPases Rac1 and Cdc42, cause X-linked intellectual disability (ID) in humans. We show here that Pix/Arhgef6 is primarily expressed in neuropil regions of the hippocampus. To study the role of Pix/Arhgef6 in neuronal development and plasticity and gain insight into the pathogenic mechanisms underlying ID, we generated Pix/Arhgef6-deficient mice. Gross brain structure in these mice appeared to be normal; however, analysis of Golgi-Cox-stained pyramidal neurons revealed an increase in both dendritic length and spine density in the hippocampus, accompanied by an overall loss in spine synapses. Early-phase long-term potentiation was reduced and long-term depression was increased in the CA1 hippocampal area of Pix/Arhgef6-deficient animals. Knockout animals exhibited impaired spatial and complex learning and less behavioral control in mildly stressful situations, suggesting that this model mimics the human ID phenotype. The structural and electrophysiological alterations in the hippocampus were accompanied by a significant reduction in active Rac1 and Cdc42, but not RhoA. -

The Mental Retardation Protein PAK3 Contributes to Synapse Formation and Plasticity in Hippocampus
10816 • The Journal of Neuroscience, December 1, 2004 • 24(48):10816–10825 Development/Plasticity/Repair The Mental Retardation Protein PAK3 Contributes to Synapse Formation and Plasticity in Hippocampus Bernadett Boda, Stefano Alberi, Irina Nikonenko, Roxanne Node-Langlois, Pascal Jourdain, Marlyse Moosmayer, Lorena Parisi-Jourdain, and Dominique Muller Department of Basic Neuroscience, Centre Medical Universitaire, 1211 Geneva 4, Switzerland Mutations of the gene coding for PAK3 (p21-activated kinase 3) are associated with X-linked, nonsyndromic forms of mental retardation (MRX) in which the only distinctive clinical feature is the cognitive deficit. The mechanisms through which PAK3 mutation produces the mental handicap remain unclear, although an involvement in the mechanisms that regulate the formation or plasticity of synaptic networks has been proposed. Here we show, using a transient transfection approach, that antisense and small interfering RNA-mediated suppression of PAK3 or expression of a dominant-negative PAK3 carrying the human MRX30 mutation in rat hippocampal organotypic slice cultures results in the formation of abnormally elongated dendritic spines and filopodia-like protrusions and a decrease in mature spine synapses. Ultrastructural analysis of the changes induced by expression of PAK3 carrying the MRX30 mutation reveals that many elongated spines fail to express postsynaptic densities or contact presynaptic terminals. These defects are associated with a reduced spontaneous activity, altered expression of AMPA-type glutamate receptors, and defective long-term potentiation. Together, these data identify PAK3 as a key regulator of synapse formation and plasticity in the hippocampus and support interpretations that these defects might contribute to the cognitive deficits underlying this form of mental retardation.