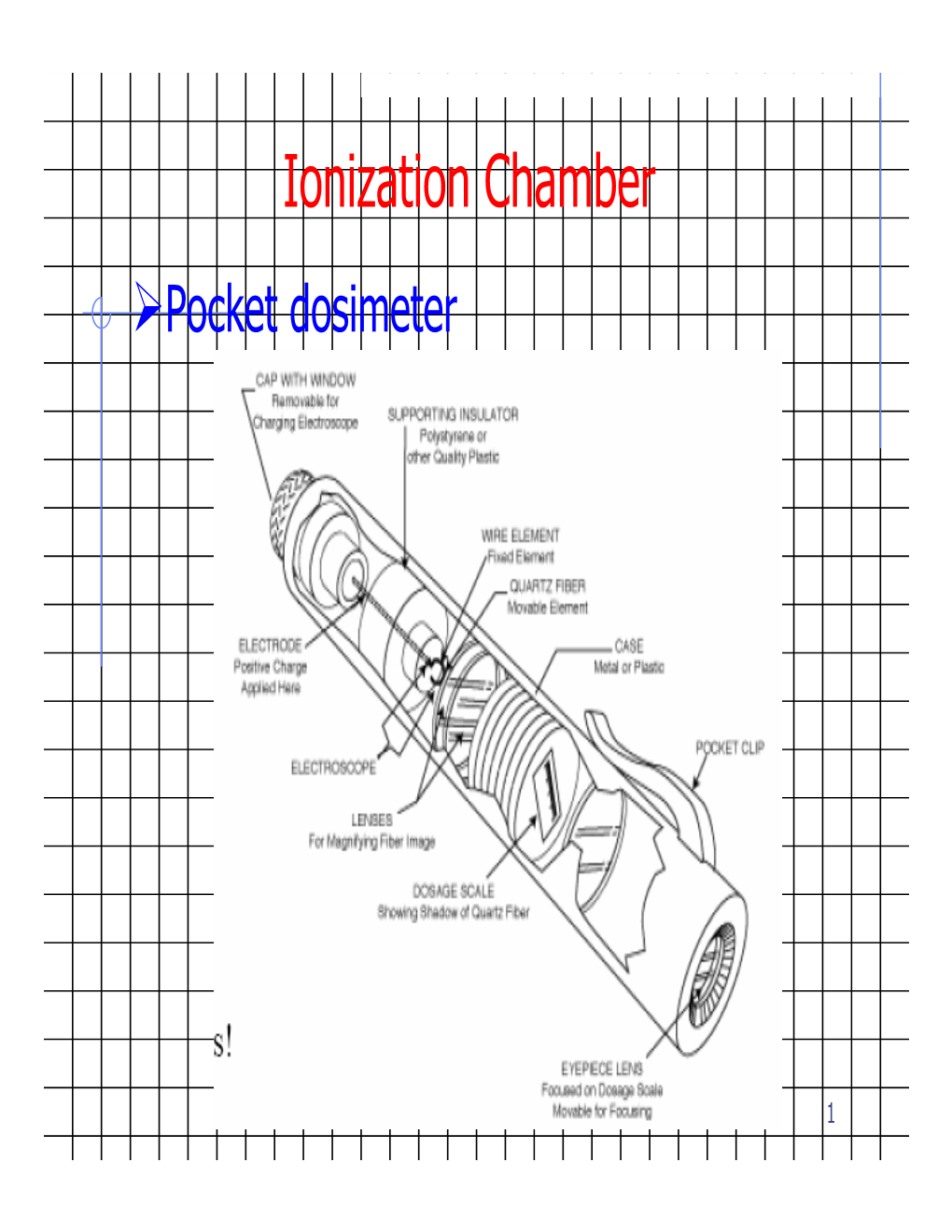
Ionization Chamber ¾Pocket Dosimeter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Low Dose Radiation Therapy for Severe COVID-19 Pneumonia: a Randomized Double- Blind Study --Manuscript Draft
International Journal of Radiation Oncology • Biology • Physics Low dose radiation therapy for severe COVID-19 pneumonia: a randomized double- blind study --Manuscript Draft-- Manuscript Number: ROB-D-21-00260R1 Article Type: Full Length Article Section/Category: Clinical Investigation - Other Corresponding Author: Alexandros Papachristofilou University Hospital Basel: Universitatsspital Basel Basel, SWITZERLAND First Author: Alexandros Papachristofilou, MD Order of Authors: Alexandros Papachristofilou, MD Tobias Finazzi, MD Andrea Blum, MD Tatjana Zehnder, MD Núria Zellweger Jens Lustenberger, MD Tristan Bauer, MD Christian Dott Yasar Avcu, M.Sc. Goetz Kohler, PhD Frank Zimmermann, MD Hans Pargger, MD Martin Siegemund, MD Abstract: Purpose The morbidity and mortality of patients requiring mechanical ventilation for coronavirus disease 2019 (COVID-19) pneumonia is considerable. We studied the use of whole- lung low dose radiation therapy (LDRT) in this patient cohort. Methods and Materials Patients admitted to the intensive care unit (ICU) and requiring mechanical ventilation for COVID-19 pneumonia were included in this randomized double-blind study. Patients were randomized to 1 Gy whole-lung LDRT or sham irradiation (sham-RT). Treatment group allocation was concealed from patients and ICU clinicians, who treated patients according to the current standard of care. Patients were followed for the primary endpoint of ventilator-free days (VFDs) at day 15 post-intervention. Secondary endpoints included overall survival, as well as changes in oxygenation and inflammatory markers. Results Twenty-two patients were randomized to either whole-lung LDRT or sham-RT between November and December 2020. Patients were generally elderly and comorbid, with a median age of 75 years in both arms. No difference in 15-day VFDs was observed between groups (p = 1.00), with a median of 0 days (range, 0-9) in the LDRT arm, and 0 days (range, 0-13) in the sham-RT arm. -

12VAC5-481-10. Definitions
Virginia Administrative Code Title 12. Health Agency 5. Department of Health Chapter 481. Virginia Radiation Protection Regulations 12VAC5-481-10. Definitions. Part I. General Provisions The following words and terms as used in this chapter shall have the following meanings unless the context clearly indicates otherwise: "A1" means the maximum activity of special form radioactive material permitted in a Type A package. This value is listed in Table 1 of 12VAC5-481-3770 F. "A2" means the maximum activity of radioactive material, other than special form radioactive material, LSA, and SCO material, permitted in a Type A package. This value is listed in Table 1 of 12VAC5-481-3770 F. "Absorbed dose" means the energy imparted by ionizing radiation per unit mass of irradiated material. The units of absorbed dose are the gray (Gy) and the rad. "Absorbed dose rate" means absorbed dose per unit time, for machines with timers, or dose monitor unit per unit time for linear accelerators. "Accelerator" means any machine capable of accelerating electrons, protons, deuterons, or other charged particles in a vacuum and of discharging the resultant particulate or other radiation into a medium at energies usually in excess of one MeV. For purposes of this definition, "particle accelerator" is an equivalent term. "Accelerator-produced material" means any material made radioactive by a particle accelerator. "Access control" means a system for allowing only approved individuals to have unescorted access to the security zone and for ensuring that all other individuals are subject to escorted access. "Accessible surface" means the external surface of the enclosure or housing of the radiation producing machine as provided by the manufacturer. -

Manual for ACRO Accreditation March 2016
American College of Radiation Oncology Manual for ACRO Accreditation March 2016 Powered by Patients First Our Focus is Radiation Oncology Safety! ACKNOWLEDGMENTS ACRO expresses its appreciation for the significant contribution and leadership of Jaroslaw Hepel, MD, FACRO, Chair of the ACRO Standards Committee, and ACRO Accreditation Medical Director, for his un- tiring efforts to bring this version of the Manual for ACRO Accreditation to publication. Thanks also go to Arve Gillette, MD, FACRO, ACRO Chancellor and a former President, and ACRO Ac- creditation Medical Director during 2011, who initiated most of the new procedures incorporated in the accreditation program when it was reintroduced after a board imposed administrative review for most of 2010. Appreciation also is expressed to Ralph Dobelbower, MD, FACRO, the founding medical director of ACRO Accreditation; Gregg Cotter, MD, FACRO, medical director after Dr. Dobelbower; and Ishmael Parsai, PhD, FACRO, physics chairman for many years; for their collective leadership in building the accreditation program from its inception in 1995. In addition, appreciation is expressed for ongoing support and commitment to the accreditation process to all the following ACRO members who have contributed, and continue to contribute, to the success of ACRO Accreditation: ACRO Executive Committee | Drs. James Welsh (President), Eduardo Fernandez (Vice President), William Rate (Secretary-Treasurer), and Arno Mundt (Chairman) ACRO Chancellors | Drs. Joanne Dragun, Gregg Franklin, Shane Hopkins, Sheila Rege, Charles Thomas, II, Harvey Wolkov, Catheryn Yashar, and Luther Brady (ex officio) ACRO Accreditation Disease Site Team Leaders | Dr. Jaroslaw Hepel (Breast Cancer); Drs. William Regine & Navesh Sharma (Gastrointestinal Cancer); Dr. Peter Orio III (Genitourinary Cancer); Dr. -

REGULATIONS and STATUTES Kansas Radiation Control Program
REGULATIONS AND STATUTES Kansas Radiation Control Program Unofficial Copy The regulations and statutes provided are for the convenience of the user but are not considered the official regulations. For more information on the official regulations, visit the Kansas Secretary of State website. For more information about official copies of statutes, visit the Kansas Legislature website. April 27, 2021 [email protected] Kansas Administrative Regulations Table of Contents Table of Contents Kansas Annotated Regulations ........................................... 2 Part 1. Definitions ............................................................... 2 Part 2. Radiation Producing Machines ............................. 67 Part 3. Licensing of Sources of Radiation ........................ 75 Part 4. Standards for Protection Against Radiation ........ 190 Part 5. Use of X-Rays in the Healing Arts ...................... 249 Part 6. Use of Sealed Radioactive Sources in the Healing Arts .............................................................................................. 291 Part 7. Special Requirements for Industrial Radiographic Operations ................................................................................... 294 Part 8. Radiation Safety Requirements for Analytical X- Ray Equipment............................................................................ 319 Part 9. Radiation Safety Requirements for Particle Accelerators ................................................................................ 323 Part 10. Notices, Instructions -

Towards Safer Radiotherapy
Patient Safety Division The Royal College of Radiologists IPEM Towards Safer Radiotherapy British Institute of Radiology Institute of Physics and Engineering in Medicine National Patient Safety Agency Society and College of Radiographers The Royal College of Radiologists Contents Foreword 4 Executive summary 5 Chapter 1 Introduction 7 Chapter 2 Nature and frequency of human errors in radiotherapy 8 Chapter 3 Defining and classifying radiotherapy errors 18 Chapter 4 Prerequisites for safe delivery of radiotherapy 24 Chapter 5 Detection and prevention of radiotherapy errors 35 Chapter 6 Learning from errors 48 Chapter 7 Dealing with consequences of radiotherapy errors 53 Summary of recommendations 58 3 Appendices Appendix 3.1 61 Appendix 4.1 69 Appendix 6.1 70 References 71 Further reading 77 Glossary of terms 78 Abbreviations 82 Membership of Working Party 84 Towards Safer Radiotherapy Foreword In my Annual Report on the State of Public Health in 2006,1 I drew attention to the problem of radiotherapy safety. Overall, radiotherapy in the United Kingdom offers a first-rate service providing high-quality care to the vast majority of patients every year. However, in a number of unfortunate cases over the last few years, overdoses of radiation led to severe harm to patients. It is recognised that these are uncommon events, yet their impact on the patient, staff involved and the wider health service are devastating. Not only does it compromise the delivery of radiotherapy, it calls into question the integrity of hospital systems and their ability to pick up errors and the capability to make sustainable changes. While further investment in radiotherapy is a continuing desirable goal, the patient safety movement has started to establish that changing the culture of an organisation involves steps more sophisticated than just investment and human resources. -

Acceptance Tests and Commissioning Measurements
Chapter 10: Acceptance Tests and Commissioning Measurements Set of 189 slides based on the chapter authored by J. L. Horton of the IAEA publication: Review of Radiation Oncology Physics: A Handbook for Teachers and Students Objective: To familiarize the student with the series of tasks and measurements required to place a radiation therapy machine into clinical operation. Slide set prepared in 2006 by G.H. Hartmann (Heidelberg, DKFZ) Comments to S. Vatnitsky: [email protected] IAEA International Atomic Energy Agency CHAPTER 10. TABLE OF CONTENTS 10.1 Introduction 10.2 Measurement Equipment 10.3 Acceptance Tests 10.4 Commissioning 10.5 Time Requirements IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 10. 1 10.1 INTRODUCTION In many areas of radiotherapy, particularly in the more readily defined physical and technical aspects of a radiotherapy unit, the term “Quality Assurance”(QA) is frequently used to summarize a variety of actions • to place the unit into clinical operation, and • to maintain its reliable performance. Typically, the entire chain of a QA program for a radiotherapy unit consists of subsequent actions as shown in the following slide. IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 10.1 Slide 1 10.1 INTRODUCTION Subsequent QA actions Purpose • Clinical needs assessment Basis for specification • Initial specification and purchase Specification of data in units of measure, process design of a tender • Acceptance testing Compliance with specifications • Commissioning for clinical use, Establishment of baseline performance (including calibration) values Monitoring the reference performance • Periodic QA tests values • Additional quality control tests after Monitoring possibly changed reference any significant repair, intervention, performance values or adjustment • Planned preventive maintenance Be prepared in case of malfunctions etc. -

Overview and Basis of Design for NCRP Report
This Report was prepared through a joint effort of NCRP Scientific Committee 46-13 on Design of Facilities for Medical Radiation Overview & Basis of Design for NCRP Therapy and AAPM Task Group 57. Report 151 James A. Deye, Chairman James E. Rodgers, Vice Chair Raymond K. Wu, Vice Chair Structural Shielding Design and Evaluation for Peter J. Biggs Patton H. McGinley Megavoltage x- and Gamma-ray Radiotherapy Facilities Richard C. McCall Liaisons Kenneth R. Kase Marc Edwards Raymond K. Wu, PhD Consultants OhioHealth Hospitals Robert O. Gorson Jeffrey H. Kleck Nisy E. Ipe Columbus, OH Secretariat Marvin Rosenstein Eric E. Kearsley Learn • Calculation methods • W, U, T, IDR, TADR, RW, Rh, • Dose at maze door • Neutron, capture gamma at door • Laminated primary barrier This Report addresses the structural shielding design New Issues since NCRP # 49 and evaluation for medical use of megavoltage x- and gamma-rays for radiotherapy and supersedes related – New types of equipment, material in NCRP Report No. 49, Structural Shielding – Some with energies above 10 MV, Design and Evaluation for Medical Use of X Rays and Gamma Rays of Energies Up to 10 MeV, which was – Many new uses for radiotherapy equipment, issued in September 1976. – Dual energy machines, The descriptive information in NCRP Report No. 49 unique to x-ray therapy installations of less than 500 – Room designs without mazes, kV (Section 6.2) and brachytherapy is not included in – Varied shielding materials including composites, this Report and that information in NCRP Report No. 49 for those categories is still applicable. – More published data on empirical methods. -

Radiation Vault Design and Shielding
Radiation Vault Design and Shielding Rajat Kudchadker, PhD Associate Professor Department of Radiation Physics NCRP Report No. 151 This report addresses the structural shielding design and evaluation for medical use of megavoltage x- and gamma- rays for radiotherapy and supersedes related material in NCRP Report No. 49, Structural Shielding Design and Evaluation for Medical Use of X Rays and Gamma Rays of Energies Up to 10 MeV, which was issued in September 1976. The descriptive information in NCRP Report No. 49 unique to x-ray therapy installations of less than 500 kV and brachytherapy is not included in this Report and that information in NCRP Report No. 49 for those categories is still applicable. Similarly therapy simulators are not covered in this report and the user is referred to the recent Report 147 for shielding of imaging facilities. NCRP Report No. 151 New Issues since NCRP # 49 • New types of equipment with energies above 10 MV • Many new uses for radiotherapy equipment • Dual energy machines and new treatment techniques • Room designs without mazes • Varied shielding materials including composites • More published data on empirical methods New Modalities New modalities include: • Cyberknife Robotic arm linacs No fixed isocenter All barriers except ceiling are primary Uses only 6 MV • Helical Tomotherapy Radiotherapy CT Uses only 6 MV Uses a beam stopper • Serial Tomotherapy MIMIC device attached to conventional linac Uses table indexer to simulate helical treatment Outdated Special Procedures New modalities include: • Intensity Modulated Radiation Therapy (IMRT) Usually at 6 MV Leakage workload >>primary, scatter workload Could be >50% of the workload on a linac • Stereotactic radiosurgery Use factors are different from 3D CRT High dose, however long setup times • Total Body Irradiation (TBI) Source of scatter is not at the isocenter Primary, leakage workload is greater than prescribed dose NCRP Report No. -

4732.0110 DEFINITIONS. Subpart 1. Scope. for Purposes of This Chapter, the Terms in This Part Have the Meanings Given Them
1 REVISOR 4732.0110 4732.0110 DEFINITIONS. Subpart 1. Scope. For purposes of this chapter, the terms in this part have the meanings given them. Subp. 2. Absorbed dose. "Absorbed dose" means the energy imparted by ionizing radiation per unit mass of irradiated material. The special unit of absorbed dose is the rad under the conventional system of measurement and is the gray under the SI system of measurement. Subp. 3. Absorbed dose rate. "Absorbed dose rate" means absorbed dose per unit time for machine with timers, or dose-monitor unit per unit time for linear accelerators. Subp. 4. Accelerator. "Accelerator" means any machine capable of accelerating electrons, protons, deuterons, or other charged particles in a vacuum and of discharging the resultant particulate or other radiation into a medium at energies usually in excess of 1 MeV. For purposes of this definition, linear accelerator, particle accelerator, and cyclotron are equivalent terms. Subp. 5. Added filtration. "Added filtration" means filtration that is in addition to the inherent filtration. Subp. 6. Adult. "Adult" means an individual 18 or more years of age or older. Subp. 7. Air kerma (K). "Air kerma (K)" means the kinetic energy released in air by ionizing radiation. Kerma is determined as the quotient of dE by dM, where dE is the sum of the initial kinetic energies of all the charged ionizing particles liberated by uncharged ionizing particles in air of mass dM. The special name for the unit of kerma is the gray (Gy). The SI unit is joule per kilogram. Subp. 8. Aluminum equivalent. "Aluminum equivalent" means the thickness of type 1100 aluminum alloy affording the same attenuation, under specified conditions, as the material in question. -

Abbreviations and Acronyms
Review of Radiation Oncology Physics: A Handbook for Teachers and Students ABBREVIATIONS AND ACRONYMS AAPM American Association of Physicists in Medicine ACR American College of Radiology ADCL Accredited Dosimetry Calibration Laboratory ALARA As low as reasonably achievable AP Anterio-posterior A-Si Amorphous silicon BAT B-mode acquisition and targeting BE Binding energy BEV Beam’s eye view B-G Bragg-Gray BGO Bismuth germanate BIPM Bureau International des Poides et Measures (France) BJR British Journal of Radiology BMT Bone marrow transplantation BNCT Boron neutron capture therapy BSF Back-scatter factor BSS Basic Safety Standards CAX Central axis CE Compton effect CEMA Converted energy per unit mass CET Coefficient of equivalent thickness CF Collimator factor CHART Continuous hyperfractionated accelerated radiation therapy CIOMS Council for International Organizations of Medical Sciences CNS Central nervous system CNT Carbon nanotube CPE Charged particle equilibrium CPU Central processing unit CSDA Continuous slowing down approximation CT Computed tomography CT-Sim CT-simulator CTV Clinical target volume DCR Digitally composited radiographs DICOM Digital imaging and communications in medicine DIN Deutches Institut für Normung (Germany) DMF Dose modifying factor DNA Deoxyribonucleic acid DRR Digitally reconstructed radiographs DVH Dose-volume histogram EBF Electron backscatter factor EM Electromagnetic EPID Electronic portal imaging device EPR Electron paramagnetic resonance ESR Electron spin resonance ESTRO European Society for Therapeutic -

Clinical Treatment Planning in External Photon Beam Radiotherapy
Chapter 7: Clinical Treatment Planning in External Photon Beam Radiotherapy Set of 232 slides based on the chapter authored by W. Parker, H. Patrocinio of the IAEA publication (ISBN 92-0-107304-6): Review of Radiation Oncology Physics: A Handbook for Teachers and Students Objective: To familiarize the student with a variety of modern photon beam radiotherapy techniques to achieve a uniform dose distribution inside the target volume and a dose as low as possible in the healthy tissues surrounding the target. Slide set prepared in 2006 by G.H. Hartmann (Heidelberg, DKFZ) Comments to S. Vatnitsky: [email protected] Version 2012 IAEA International Atomic Energy Agency CHAPTER 7. TABLE OF CONTENTS 7.1 Introduction 7.2 Volume Definition 7.3 Dose Specification 7.4 Patient Data Acquisition and Simulation 7.5 Clinical Considerations for Photon Beams 7.6 Treatment Plan Evaluation 7.7 Treatment Time and Monitor Unit Calculations IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 7.Slide 1 7.1 INTRODUCTION General considerations for photon beams Almost a dogma in external beam radiotherapy: Successful radiotherapy requires a uniform dose distribution within the target (tumor). External photon beam radiotherapy is usually carried out with multiple radiation beams in order to achieve a uniform dose distribution inside the target volume and a dose as low as possible in healthy tissues surrounding the target. IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 7.1 Slide 1 7.1 INTRODUCTION Criteria of a uniform dose distribution within the target Recommendations regarding dose uniformity, prescribing, recording, and reporting photon beam therapy are set forth by the International Commission on Radiation Units and Measurements (ICRU). -

QUALITY CONTROL of MODERN LINEAR ACCELERATOR: DOSE STABILITY LONG and SHORT-TERM Md Tofiz Uddin, National Institute of Cancer Research and Hospital, Dhaka, Bangladesh
WEPPD061 Proceedings of IPAC2012, New Orleans, Louisiana, USA QUALITY CONTROL OF MODERN LINEAR ACCELERATOR: DOSE STABILITY LONG AND SHORT-TERM Md Tofiz Uddin, National Institute of Cancer Research and Hospital, Dhaka, Bangladesh Abstract reference instrument. The measurements are carried out Quality Control (QC) data of modern linear with relatively long time intervals since they are workful accelerators, collected by National Institute of Cancer and time consuming. Research and Hospital, Dhaka, Bangladesh between the Approximated constancy checks (CC) of output are years 2006 and 2010, were analyzed. Three Linear carried out more often by using by fast and easily Accelerators is included in this work. The goal was to movable equipment. A CC device may not be very stable provide information for the evaluation and elaboration of requiring regular calibrations against the local reference QC of accelerator outputs and to propose a method for instrument [4]. The use of an appropriate QC procedure QC data analysis. Short- and long-term drifts in outputs for output should maximize the detection of both ‘normal’ were quantified by fitting empirical mathematical models and unexpected changes in output levels. QC programs to the QA measurements. Normally, long-term drifts were given by different international or national authorities well (≤1.5%) modeled by either a straight line or a single- have recommended considerable variable time intervals exponential function. A drift of 2% occurred in 18 ± 12 for the output measurements ranging from one week to months. The shortest drift times of only 2–3 months were even one year. Intuitively it is safer to have short output observed for some new accelerators just after the measurement intervals but too redundant measurements commissioning but they stabilized during the first 2–3 waste resources and time that could be used for treatments years.