CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coimbatore Commissionerate Jurisdiction
Coimbatore Commissionerate Jurisdiction The jurisdiction of Coimbatore Commissionerate will cover the areas covering the entire Districts of Coimbatore, Nilgiris and the District of Tirupur excluding Dharapuram, Kangeyam taluks and Uthukkuli Firka and Kunnathur Firka of Avinashi Taluk * in the State of Tamil Nadu. *(Uthukkuli Firka and Kunnathur Firka are now known as Uthukkuli Taluk). Location | 617, A.T.D. STR.EE[, RACE COURSE, COIMBATORE: 641018 Divisions under the jurisdiction of Coimbatore Commissionerate Sl.No. Divisions L. Coimbatore I Division 2. Coimbatore II Division 3. Coimbatore III Division 4. Coimbatore IV Division 5. Pollachi Division 6. Tirupur Division 7. Coonoor Division Page 47 of 83 1. Coimbatore I Division of Coimbatore Commissionerate: Location L44L, ELGI Building, Trichy Road, COIMBATORT- 641018 AreascoveringWardNos.l to4,LO to 15, 18to24and76 to79of Coimbatore City Municipal Corporation limit and Jurisdiction Perianaickanpalayam Firka, Chinna Thadagam, 24-Yeerapandi, Pannimadai, Somayampalayam, Goundenpalayam and Nanjundapuram villages of Thudiyalur Firka of Coimbatore North Taluk and Vellamadai of Sarkar Samakulam Firka of Coimbatore North Taluk of Coimbatore District . Name of the Location Jurisdiction Range Areas covering Ward Nos. 10 to 15, 20 to 24, 76 to 79 of Coimbatore Municipal CBE Corporation; revenue villages of I-A Goundenpalayam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore 5th Floor, AP Arcade, District. Singapore PIaza,333 Areas covering Ward Nos. 1 to 4 , 18 Cross Cut Road, Coimbatore Municipal Coimbatore -641012. and 19 of Corporation; revenue villages of 24- CBE Veerapandi, Somayampalayam, I-B Pannimadai, Nanjundapuram, Chinna Thadagam of Thudiyalur Firka of Coimbatore North Taluk of Coimbatore District. Areas covering revenue villages of Narasimhanaickenpalayam, CBE Kurudampalayam of r-c Periyanaickenpalayam Firka of Coimbatore North Taluk of Coimbatore District. -

TAMILNADU NAME of the DISTRICT : CHENNAI Division: Thiruvanmiyur 1 Hotel Saravana Bhavan Hotel Saravana Bhavan, Perungudi, Chennai-96
DETAILS OF DHABA'S IN TAMILNADU NAME OF THE DISTRICT : CHENNAI Division: Thiruvanmiyur 1 Hotel Saravana Bhavan Hotel Saravana Bhavan, Perungudi, Chennai-96. 7823973052 2 Hotel Hot Chips Hotel Hot Chips, ECR Road, Chennai-41 044-2449698 3 Yaa Moideen Briyani Yaa Moideen Briyani, ECR Road, Chennai-41 044-43838315 4 Kuppana Hotel Junior Kuppana, OMR, Chennai-96 044-224545959 Sree Madurai Devar Hotel, Porur Toll-8, NH Road 5 Sree Madurai Devar Hotel 72993 87778 Porur, Toll Gate Vanagarm, Porur, Chennai. Hotel Madurai Pandiyan, Porur Toll No.49, Bye Pass 6 Hotel Madurai Pandiyan road, Om sakthi nager, Maduravoyal, NR Tool Gate, 98841 83534 Chennai-95. Briyani Dream Porur Toll-39, Om Sakthi Nager, Porur 7 Briyani Dream 75500 60033 road, Chennai-95. Hotel Bypass Orient Porur Toll Bo.12B, Swami 8 Hotel BypassOrient 98411 92606 Vivekandar road bypass, Chennai-116 District: KANCHIPURAM Division : Kanchipuram New Panjabi Dhaba, Chennai to Bengalure Highway, 9 Rajendiran 9786448787 Rajakulam, Kanchipuram New Punjabi Dhaba, Chennai to Bengalure Highway, 10 Rajendiran 9786448787 Vedal, Kanchipuram, 9080772817 11 Punjab Dhaba Punjabi Dhaba, White Gate, Kanchipuram 9600407219 12 JP Hotels J P Hotels, Baluchettichatram, Kanchipuram, Hotel Sakthi Ganapathi, White Gate, Chennai to 13 Sakthi Ganapathi Hotel 9003855555 Bengalure Highway, Kanchipuram Hotel Ramanas, Chennai to Bengalure Highway, 14 Guru 9443311222 Kilambi, Kanchipuram Division: TAMBARAM AL-Taj Hotel, GST Road, Peerkan karanai, Chennai- 15 K.Thameem Ansari 9840687210 63 Division: SRIPERUMBUTHUR -

Gobichettipalayam
GOBICHETTIPALAYAM S.No. ROLL No. NAME OF ADVOCATE ADDRESS MEVANI VILLAGE, GOBICHETTIPALAYAM 1 453/1990 ANANDAN M. TALUK, ERODE DIST, PIN - 638313 SELLAKUMARAPALAYAM, 2 528/2011 ANDAVAN S.K. POLAVAKALIPALAYAM PO. GOBI TK. ERODE DT. 638476 27, KANNIKA PARAMESHWARI STREET, 3 1690/1999 ARAVINDAN K. GOBICHETTIPALAYAM, ERODE 70/A, GANDHIPURAM, L. KALLIPATTI, GOBI, 4 3149/2013 ARCCHANA R. ERODE 638452 120,VIVEKANANDAR STREET,SAKTHI NAGAR 5 1185/1997 AYYASAMY K. VIA,P.METTUPALAYAM,BHAVANI TALUK,ERODE-638315 NO.28, BAZAAR ST, GOBICHETTIPALAYAM, 6 1040/1996 BALAMURUGAN G.J. ERODE-638452 NO.29, K.P. EXTENSION, 7 662/1981 BALASUBRAMANIAN G.R. GOBICHETTIPALAYAM. 4/188, M.L.N.HOUSE ARASUR & PO, 8 2068/2000 BALASUBRAMANIAN N. SATHYAMANGALAM TK, ERODE DIST 638454 ANGAYA GOUNDER STREET, KALLIPATTI 9 1412/2013 BARANEEDHARAN K.V. POST, GOBI TALUK, ERODE DIST - 638 505 NO.1, UPPU KIDANGU STREET, 10 879/1995 BASKARAN K.S. GOBICHETTIPALAYAM,ERODE NO.27, CANAL STREET, GOBICHETTIPALAYAM, 11 439/1980 BHARATHI G.K. ERODE - 638452 65/25,PATHIVILAS 12 1877/2000 BIRUNTHA I. STREET,GOBICHETTIPALAYAM,ERODE S.No. ROLL No. NAME OF ADVOCATE ADDRESS 22, AGRAHARA STREET, PERIYA KODIVERI 13 1932/2003 BOOPATHY K.T (PO),SATHYAMANGALAM,ERODE DT-638503. 176, POOSARI VALASU, 14 1893/2001 CHANDRAMOHAN B. PALAVAKALIAPALAYAM POST,GOBICHETTIPALAYAM-638476 DOOR NO:16-B,CKS NAGAR,OPP COURT 15 915/1997 CHANDRASEKARAN K. CAMPUS,GOBICHETTIPALAYAM-638452 NO:20, 4TH STREET MAN NAGAR 16 167/1979 CHINNASWAMY K.S. GOBICHETTIPALAYAM ERODE DIST -638452 D.NO. 254/1, KAMARAJ STREET, D.G.PUDUR 17 1645/2007 CHITHAMBARAM P. PO, NALL ROAD, GOBICHETTI PALAYAM TALUK. -
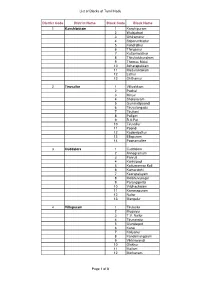
List of Blocks of Tamil Nadu District Code District Name Block Code
List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 1 Kanchipuram 1 Kanchipuram 2 Walajabad 3 Uthiramerur 4 Sriperumbudur 5 Kundrathur 6 Thiruporur 7 Kattankolathur 8 Thirukalukundram 9 Thomas Malai 10 Acharapakkam 11 Madurantakam 12 Lathur 13 Chithamur 2 Tiruvallur 1 Villivakkam 2 Puzhal 3 Minjur 4 Sholavaram 5 Gummidipoondi 6 Tiruvalangadu 7 Tiruttani 8 Pallipet 9 R.K.Pet 10 Tiruvallur 11 Poondi 12 Kadambathur 13 Ellapuram 14 Poonamallee 3 Cuddalore 1 Cuddalore 2 Annagramam 3 Panruti 4 Kurinjipadi 5 Kattumannar Koil 6 Kumaratchi 7 Keerapalayam 8 Melbhuvanagiri 9 Parangipettai 10 Vridhachalam 11 Kammapuram 12 Nallur 13 Mangalur 4 Villupuram 1 Tirukoilur 2 Mugaiyur 3 T.V. Nallur 4 Tirunavalur 5 Ulundurpet 6 Kanai 7 Koliyanur 8 Kandamangalam 9 Vikkiravandi 10 Olakkur 11 Mailam 12 Merkanam Page 1 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 13 Vanur 14 Gingee 15 Vallam 16 Melmalayanur 17 Kallakurichi 18 Chinnasalem 19 Rishivandiyam 20 Sankarapuram 21 Thiyagadurgam 22 Kalrayan Hills 5 Vellore 1 Vellore 2 Kaniyambadi 3 Anaicut 4 Madhanur 5 Katpadi 6 K.V. Kuppam 7 Gudiyatham 8 Pernambet 9 Walajah 10 Sholinghur 11 Arakonam 12 Nemili 13 Kaveripakkam 14 Arcot 15 Thimiri 16 Thirupathur 17 Jolarpet 18 Kandhili 19 Natrampalli 20 Alangayam 6 Tiruvannamalai 1 Tiruvannamalai 2 Kilpennathur 3 Thurinjapuram 4 Polur 5 Kalasapakkam 6 Chetpet 7 Chengam 8 Pudupalayam 9 Thandrampet 10 Jawadumalai 11 Cheyyar 12 Anakkavoor 13 Vembakkam 14 Vandavasi 15 Thellar 16 Peranamallur 17 Arni 18 West Arni 7 Salem 1 Salem 2 Veerapandy 3 Panamarathupatti 4 Ayothiyapattinam Page 2 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 5 Valapady 6 Yercaud 7 P.N.Palayam 8 Attur 9 Gangavalli 10 Thalaivasal 11 Kolathur 12 Nangavalli 13 Mecheri 14 Omalur 15 Tharamangalam 16 Kadayampatti 17 Sankari 18 Idappady 19 Konganapuram 20 Mac. -

District Survey Report Tiruppur District
DISTRICT SURVEY REPORT TIRUPPUR DISTRICT DISTRICT ENVIRONMENT IMPACT ASSESSMENT AUTHORITY (DEIAA), TIRUPPUR AUGUST 2017 1 DISTRICT SURVEY REPORT TIRUPPUR DISTRICT CONTENT Chapter Page No. 1. Introduction 01 2. Overview of mining activity 03 3. The l ist of Mining Lease details 05 4. Details of Royalty / Revenue received in last three years (2014 -15 43 to 2016-17) 5. Details of production of sand / Bajari / minor minerals in the last 43 three years (2014-15 to 2016-17) 6. Processes of d eposition of sediment s in the rivers of the district 44 7. General profile of the District 49 8. Land utilisation pattern in the District 51 9. Physiography of the District 53 10. Rainfall data month-wise 55 11 . Geology and Mineral wealth of the Distr ict 56 11.1. An outline on Geology of Tamilnadu 56 11.2. Geology of Tiruppur District 58 11.3. Stratigraphy of the area 58 11.4.Mineral occurrences in Tiruppur District 59 11.4.1 Rough Stone (Charnockite and Granite Gneiss) 60 11.4.2. Dimensional stone-Granite Varieties 65 11.4.2.1 Nepheline Syenite 11.4.2.2 Quartzo Feldspathic Gneiss 11.4.3. Magnesite and Dunite 66 11.4.4. Gypsum 67 11.4.5. Kankar 68 11.4.6. Quartz and Feldspar 69 11.4.7. River Sand 71 11.4.8. Gravel and Silt 71 12 . Conclusion and Recommendations 72 2 LIST OF PLATES Plate No. Page No. Plate1. A. Schematic diagram of process on meander bend 45 Plate1. B. Meandering of Amaravathi River, Near Veerachimangalam, Tiruppur 45 district. -
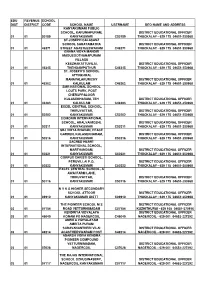
Deo &Ceo Address List
EDU REVENUE SCHOOL DIST DISTRICT CODE SCHOOL NAME USERNAME DEO NAME AND ADDRESS KANYAKUMARI PUBLIC SCHOOL, KARUNIAPURAM, DISTRICT EDUCATIONAL OFFICER 01 01 50189 KANYAKUMARI C50189 THUCKALAY - 629 175 04651-250968 ST.JOSEPH CALASANZ SCHOOL SAHAYAMATHA DISTRICT EDUCATIONAL OFFICER 01 01 46271 STREET AGASTEESWARAM C46271 THUCKALAY - 629 175 04651-250968 GNANA VIDYA MANDIR MADUSOOTHANAPURAM VILLAGE KEEZHAKATTUVILAI, DISTRICT EDUCATIONAL OFFICER 01 01 46345 THENGAMPUTHUR C46345 THUCKALAY - 629 175 04651-250968 ST. JOSEPH'S SCHOOL ATTINKARAI, MANAVALAKURICHY DISTRICT EDUCATIONAL OFFICER 01 01 46362 KALKULAM C46362 THUCKALAY - 629 175 04651-250968 SMR NATIONAL SCHOOL LOUTS PARK, POST CHERUPPALOOR KULASEKHARAM, TEH DISTRICT EDUCATIONAL OFFICER 01 01 46383 KALKULAM C46383 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER 01 01 50202 KANYAKUMARI C50202 THUCKALAY - 629 175 04651-250968 COMORIN INTERNATIONAL SCHOOL, ARALVAIMOZHI, DISTRICT EDUCATIONAL OFFICER 01 01 50211 KANYAKUMARI C50211 THUCKALAY - 629 175 04651-250968 SBJ VIDYA BHAVAN, PEACE GARDEN, KULASEKHARAM, DISTRICT EDUCATIONAL OFFICER 01 01 50216 KANYAKUMARI C50216 THUCKALAY - 629 175 04651-250968 SACRED HEART INTERNATIONAL SCHOOL, MARTHANDAM, DISTRICT EDUCATIONAL OFFICER 01 01 50221 KANYAKUMARI C50221 THUCKALAY - 629 175 04651-250968 CORPUS CHRISTI SCHOOL, PERUVILLAI P.O, DISTRICT EDUCATIONAL OFFICER 01 01 50222 KANYAKUMARI C50222 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, A AWAI FARM LANE, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER -

Rural Fi Bank Mitra List -Tamilnadu State
RURAL FI BANK MITRA LIST -TAMILNADU STATE NAME OF THE NAME OF THE NAME OF THE NAME OF THE BRANCH BRANCH NAME OF THE VILLAGE GENDER S.NO NAME OF THE BRANCH BANK MITRA NAME MOBILE NUMBER STATE DISTRICT TALUK DIVISION CODE CATEGORY POINT (F/M) 1 TAMILNADU TIRUVANNAMALAI ARNI VILLUPURAM ARNI 1108 SEMI URBAN PUDUPATTU USHA M 7708309603 THIMMARASANAICKAN 2 TAMILNADU THENI AUNDIPATTY MADURAI AUNDIPATTY 1110 SEMI URBAN MURUGASEN V M 9600272581 UR/ 3 TAMILNADU THENI AUNDIPATTY MADURAI AUNDIPATTY 1110 SEMI URBAN POMMINAYAKANPATTI BALANAKENDRAN C M 9092183546 4 TAMILNADU DINDIGUL NEELAKOTTAI KARUR BATLAGUNDU 1112 SEMI URBAN OLD BATLAGUNDU ARUN KUMAR D M 9489832341 5 TAMILNADU ERODE BHAVANI KARUR BHAVANI 1114 SEMI URBAN ANDIKULAM RAJU T M 8973317830 6 TAMILNADU ERODE CHENNIMALAI KARUR CHENNIMALAI 1641 SEMI URBAN ELLAIGRAMAM KULANDAVEL R G M 9976118370 7 TAMILNADU ERODE CHENNIMALAI KARUR CHENNIMALAI 1641 SEMI URBAN KUPPUCHIPALAYAM SENTHIL M 8344136321 8 TAMILNADU CUDDALORE CHIDAMBARAM VILLUPURAM CHIDAMBRAM 1116 SEMI URBAN C.THANDESWARANALLURTHILAGAVATHI C F 9629502918 9 TAMILNADU DINDIGUL CHINNALAPATTI MADURAI CHINNALAPATTI 1117 SEMI URBAN MUNNILAKOTTAI NAGANIMMI F 8883505650 10 TAMILNADU THENI UTHAMAPALAYAM MADURAI CHINNAMANUR 1118 SEMI URBAN PULIKUTHI ESWARAN M 9942158538 11 TAMILNADU THENI CHINNAMANUR MADURAI CHINNAMANUR 1118 SEMI URBAN MARKEYANKOTTAI BHARATHI V F 9940763670 12 TAMILNADU TIRUPPUR DHARAPURAM KARUR DHARAPURAM 1126 SEMI URBAN MADATHUPALAYAM GANDHIMATHI A F 9843912225 13 TAMILNADU TIRUPPUR DHARAPURAM KARUR DHARAPURAM 1126 SEMI URBAN -

Farm Machine Dealers - Tamilnadu (------All Districts ------)
FARM MACHINE DEALERS - TAMILNADU (------ ALL DISTRICTS ------) Sl.No Name and address Products Contacts 1 Sri Venkateswara Agencies Diesel engines, Paddy Ph: 04112222172 Address: 79-A,Nellukkara Transpalnter, Power tiller, Rice Mob: 9443349922 Street,Kanchipuram-681502 transplanter, Self propelled Web: power weeder, Self propelled [email protected] reaper,Self-propelled paddy m transplanter, Self propelled rice transplanter, Trolley 2 Madras Farm Eqipments Agricultural implements, Cage Mob: 9443521489 Address: No.3, GST Road, wheel, Diesel engines, Heavy Web: Madurantakam - 603 306 duty adjustable tiller, Medium http://www.shrachi.c duty tiller, Power operated om/btl_agro/index. reaper, Power tiller 3 Sri Vinayaga tillers Agricultural implements, Cage Mob: 9944125848 Address: wheel, Diesel engines, Heavy Web: MapuramVillage,Vandavasi duty adjustable tiller, Medium http://www.shrachi.c Road Madurantakam (tk) duty tiller, Power operated om/btl_agro/index.p Kanchipuram reaper, Power tiller hp 4 Tamil Nadu Agro Service Diesel engines, Power tiller Mob: 9842630121, Address: No. 13, East Raja 9842652543. Street, Kanchipuram 5 M/S. Sri Venkateshwara Power tiller Mob: 9443540227 Power Tiller Agencies Address: No. 5, Bus Stand, At P.O Thirukalukundram – 603 109, Dist- Kanchipuram, 6 Bhavani Agro Service Power operated reaper, Power Ph:41112223104 Address: 103,Vanigar Street, tiller Mob: 9894615088 Kanchipuram 7 Ayyan Agro Services Brush cutter, Power tiller, Rice Mob: 9944725059 Address: 10/140 Periya transplanter TheruTirukulam T.K, Kanchipuram 8 Aiswarya Agro Service Brush cutter, Power tiller, Rice Mob: 9944725059 Address:38,B/1 Salai Street transplanter kanchipuram 9 Need Services Chaff cutter Mob: 9443069453 Address: Plot 14, Ayyan Karunai Appar Street, Saravana Avenue, Kannivakkam, Kanchiuram 10 Arasu Agro Services Cage wheel, Cultivator, Disc Mob: 9445393864 Address: shop.no.4, plough Aadhiparasakthi commercial complex, G.S.T. -

RODATA TN.Pdf
Details in subsequent pages are as on 01/04/12 For information only. In case of any discrepancy, the official records prevail. DETAILS OF THE DEALERSHIP OF HPCL TO BE UPLOADED IN THE PORTAL SOUTH ZONE SR. No. Regional Office State Name of dealership Dealership address (incl. location, Dist, State, PIN) Name(s) of Proprietor/Partner(s) Outlet Telephone No. 1 CHENNAI RO Tamilnadu (AJ Service Station Adhoc) No. 32, Thyagaraya Road, T Nagar, Chennai - 600 017 COCO - Not applicable 044-42147828 No.85-A ARUMUGANAR SALAI, TIRUPATTUR-VELLORE 2 CHENNAI RO Tamilnadu A G S AGENCIES Smt. S. MYTHILI 04179220006 DISTRICT, PIN:635601. No.332/2, Alappakkam Main Road, Alapakkam, Porur, Chennai - 3 CHENNAI RO Tamilnadu A G Saratha Agency S.JAIKUMAR 044-24766925 600 116. 4 CHENNAI RO Tamilnadu Abitha Agency, Kanji koot Road, Thiruvannamalai Dt, Kanji - 606 702 S.POONGUDAI 9443226478 5 CHENNAI RO Tamilnadu Agni Agency No. 15, R.K. Mutt Road, Mylapore, Chennai - 600 004 V.P.Sridhar 044-42102411 6 CHENNAI RO Tamilnadu Agro Service NH46, Madanur - 8. QUASI-GOVERNMENT CO-OPERATIVE SOCIETY NO LANDLINE NUMBER 7 CHENNAI RO Tamilnadu AJ Service Station No. 32, Thyagaraya Road, T Nagar, Chennai - 600 017 A R Kamalan & Preetha Kamalan 044-24347277 No. 242, Royapettah High Road, Royapettah, Chennai - 600 8 CHENNAI RO Tamilnadu Anna Auto Drivers Co-operative Society Ltd SECRETORY - P Jairaj 044-28132045 014. 9 CHENNAI RO Tamilnadu Anna Nagar Auto No.E-100, 3rd Avenue Anna Nagar, Chennai - 600 102 Mr J.Antony 044-26215405 No. 1A, Lakshmi Nagar, Moondram Kattalai, Kundrathur Main 10 CHENNAI RO Tamilnadu Annai Madha Enterprises M/s. -

Khadi Institution Profile Khadi and Village
KHADI AND VILLAGE INDUSTRIES COMISSION KHADI INSTITUTION PROFILE Office Name : SO CHENNAI TAMIL NADU Institution Code : 689 Institution Name : COIMBATORE SOUTH SARVODAYA SANGH Address: : 7,NEW BEEMAR AGRAHARAM, Post : DHARAPURAM City/Village : DHARAPURAM Pincode : 638656 State : TAMIL NADU District : TIRIPUR Aided by : KVIC District : B Contact Person Name Email ID Mobile No. Chairman : N VELUSAMY [email protected] 8903460305 Secretary : K PARVATHI [email protected] 9787721079 Nodal Officer : Registration Detail Registration Date Registration No. Registration Type 01-01-1111 324/2011 SOC Khadi Certificate No. TND/1582 Date : 31-MAR_2021 Khadi Mark No. KVIC/CKMC/TN-053 Khadi Mark Dt. 09-Oct-2014 Sales Outlet Details Type Name Address City Pincode Production cum Sales NOT WORKING - CLOSED THOTTIPALAYAM TIRUPUR 638656 Outlet PRODUCTION CENTRE 3/138, THERPATTI DHARAPURAM 638712 Production cum Sales PRODUCTION CUM SALES 33, NEW BEERMAR DHARAPURAM 638656 Outlet CENTER STREET Sales Outlet KHADI GRAMODYOG SALES 246, VASANTHA DHARAPURAM 638656 ROAD, Sales Outlet KHADI GRAMODYOG 7, NEW BEEMAR DHARAPURAM 688656 BHAVAN AGRAHARAM Production cum Sales PRODUCTION CUM SALES 59, MAMBADI 638106 Outlet CENTER AKKARAIPALAYAM Sales Outlet NOT WORKING - CLOSED GRAMASHILPA GANAPATHY 638656 SATHI ROAD , Sales Outlet NOT WORKING - CLOSED DHALAVAIPATTINA DHALAVAIPATTINA 638672 M, M Production cum Sales NOT WORKING - CLOSED D.NO. 33, NEW DHARAPURAM 638656 Outlet BEEMAR AGRAHARAM Production cum Sales NOT WORKING - CLOSED 59, MULANUR 638106 Outlet AKKARAIPALAYAM Sales Outlet NOT WORKING - CLOSED D.NO. 7, BEEMAR DHARAPURAM 638656 AGRAHARAM Infrastructure Details 05 October 2021 Page 1 of 3 Infrastructure Type Description in No. Remarks CHARKHA 6 Spindle Charkha 104 Loom Traditional Loom 54 Land Details Structure Land / Building Market Value Street Village District Area Details (in Rs.) 7,NEW BEEMAR MAMBADI VILL,ERODE. -

Tamil Nadu 425 SEIAA Meeting AGENDA Venue
State Environment Impact Assessment Authority (SEIAA) Tamil Nadu 425 SEIAA Meeting AGENDA Venue: SEIAA Office Please Check MoEF&CC Website at www.parivesh.nic.in for details and updates From Date:15 Feb 2021 TO Date:15 Feb 2021 CONSIDERATION/RECONSIDERATION OF ENVIRONMENTAL CLEARANCE S.No Proposal Earth Quarry of Thiru.N.Chinnadurai S. State District Tehsil Village (1) No. (1.) Tamil Nadu Tirunelveli Sivagiri Dharugapuram [SIA/TN/MIN/136470/2020 , 7713 ] Rough stone and Gravel quarry of M. Rajamuniyasamy S. State District Tehsil Village (2) No. (1.) Tamil Nadu Virudhunagar Vembakottai Nathikudi [SIA/TN/MIN/149327/2020 , 7531 ] R.Ramar, Earth quarry project over an Extent of 2.05.0Ha in S.F.No. 49/3 (P) at Thoppampatty Village of Andipatti Taluk, Theni District. S. State District Tehsil Village (3) No. (1.) Tamil Nadu Theni Andipatti Thoppampatty [SIA/TN/MIN/174010/2020 , 7886 ] Rough Stone and Gravel Quarry of Thiru.D.Gnanasekaran, Survey Nos.1/4 and 2/1 over an area of 2.15.0Ha in Nagalapuram Village, Thuraiyur Taluk, Tiruchirappalli District S. State District Tehsil Village (4) No. NAGALAPURAM (1.) Tamil Nadu Tiruchirappalli Thuraiyur VILLAGE [SIA/TN/MIN/174507/2020 , 7911 ] ROUGH STONE AND GRAVEL QUARRY OF THIRU.P.MOORTHY AT (5) SURVEY NO. 1204 OVER AN AREA OF 1.65.5Ha IN KUNNUR VILLAGE, ANDIPATTI TALUK, THENI DISTRICT S. State District Tehsil Village No. KUNNUR (1.) Tamil Nadu Theni Andipatti VILLAGE [SIA/TN/MIN/175706/2020 , 7912 ] ROUGH STONE QUARRY OF THIRU.A.IRUDHAYA JEYAKUMAR, AT SURVEY NO.198/1A1 OVER AN AREA OF 2.43.0HA IN MUKKUDAL VILLAGE, CHERANMAHADEVI TALUK, TIRUNELVELI DISTRICT S. -

“Korangadu” – Traditional Dryland Grass Farming System in Semi Arid
II. Indigenous Coping Mechanism by Farmers and Livestock Keepers against Mortality of Cattle, Fodder Development and making animal husbandry as Profitable Activity 1. “Korangadu” – Traditional Dryland Grass Farming System to Earn Income in Drylands Intoduction : “Korangadu” is a traditional pastureland farming system existing in semi arid tract of Tamil Nadu state of South India viz., Dharapuram, Kangeyam, Palladam, Moolanur and Kallimanthayam areas. This region receives annual rainfall of 600 – 675 mm. The soil is laterite red soil or with gravel type and water will not stagnate on any amount of rainfall. The region situates in rain shadow region of westernghats. The majority of the rural population depends upon livestock; they are settled agro pastoralsits and allow their animals to graze in their own grassland paddocks confined to 2 – 4 ha size. Korangadu has predominantly 3 major species of flora which are spatially in 3 tiers. The lower tier is grown with grass Cenchrus ; tree species include Acacia leucophloea locally called as Velvel and land is fenced with thorny shrub locally called as Kiluvai (Commiphora berryii) as live fence. Korangadu is owned privately by individual farmers and it is roughly estimated that there are about more than 50,000 individuals keeping their own paddocks of about 1-2 ha size of land. Approximately 50,000 ha. of Korangadu pasture land is noticed in 500 villages in Erode, Karur, Dindigul and Coimbatore Districts of Tamil Nadu State, Southern India. The size of individual paddocks of Korangadu land ranges from 1.5 ha to 10 ha depending upon the wealth status or ownership pattern by farmers.