Coral Histopathology II
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Polyp and the Medusa Life on the Move
The Polyp and the Medusa Life on the Move Millions of years ago, unlikely pioneers sparked a revolution. Cnidarians set animal life in motion. So much of what we take for granted today began with Cnidarians. FROM SHAPE OF LIFE The Polyp and the Medusa Life on the Move Take a moment to follow these instructions: Raise your right hand in front of your eyes. Make a fist. Make the peace sign with your first and second fingers. Make a fist again. Open your hand. Read the next paragraph. What you just did was exhibit a trait we associate with all animals, a trait called, quite simply, movement. And not only did you just move your hand, but you moved it after passing the idea of movement through your brain and nerve cells to command the muscles in your hand to obey. To do this, your body needs muscles to move and nerves to transmit and coordinate movement, whether voluntary or involuntary. The bit of business involved in making fists and peace signs is pretty complex behavior, but it pales by comparison with the suites of thought and movement associated with throwing a curve ball, walking, swimming, dancing, breathing, landing an airplane, running down prey, or fleeing a predator. But whether by thought or instinct, you and all animals except sponges have the ability to move and to carry out complex sequences of movement called behavior. In fact, movement is such a basic part of being an animal that we tend to define animalness as having the ability to move and behave. -

Cassiopea Xamachana (Upside-Down Jellyfish)
UWI The Online Guide to the Animals of Trinidad and Tobago Ecology Cassiopea xamachana (Upside-down Jellyfish) Order: Rhizostomeae (Eight-armed Jellyfish) Class: Scyphozoa (Jellyfish) Phylum: Cnidaria (Corals, Sea Anemones and Jellyfish) Fig. 1. Upside-down jellyfish, Cassiopea xamachana. [http://images.fineartamerica.com/images-medium-large/upside-down-jellyfish-cassiopea-sp-pete-oxford.jpg, downloaded 9 March 2016] TRAITS. Cassiopea xamachana, also known as the upside-down jellyfish, is quite large with a dominant medusa (adult jellyfish phase) about 30cm in diameter (Encyclopaedia of Life, 2014), resembling more of a sea anemone than a typical jellyfish. The name is associated with the fact that the umbrella (bell-shaped part) settles on the bottom of the sea floor while its frilly tentacles face upwards (Fig. 1). The saucer-shaped umbrella is relatively flat with a well-defined central depression on the upper surface (exumbrella), the side opposite the tentacles (Berryman, 2016). This depression gives the jellyfish the ability to stick to the bottom of the sea floor while it pulsates gently, via a suction action. There are eight oral arms (tentacles) around the mouth, branched elaborately in four pairs. The most commonly seen colour is a greenish grey-blue, due to the presence of zooxanthellae (algae) embedded in the mesoglea (jelly) of the body, and especially the arms. The mobile medusa stage is dioecious, which means that there are separate males and females, although there are no features which distinguish the sexes. The polyp stage is sessile (fixed to the substrate) and small (Sterrer, 1986). UWI The Online Guide to the Animals of Trinidad and Tobago Ecology DISTRIBUTION. -

PROGRAMME ABSTRACTS AGM Papers
The Palaeontological Association 63rd Annual Meeting 15th–21st December 2019 University of Valencia, Spain PROGRAMME ABSTRACTS AGM papers Palaeontological Association 6 ANNUAL MEETING ANNUAL MEETING Palaeontological Association 1 The Palaeontological Association 63rd Annual Meeting 15th–21st December 2019 University of Valencia The programme and abstracts for the 63rd Annual Meeting of the Palaeontological Association are provided after the following information and summary of the meeting. An easy-to-navigate pocket guide to the Meeting is also available to delegates. Venue The Annual Meeting will take place in the faculties of Philosophy and Philology on the Blasco Ibañez Campus of the University of Valencia. The Symposium will take place in the Salon Actos Manuel Sanchis Guarner in the Faculty of Philology. The main meeting will take place in this and a nearby lecture theatre (Salon Actos, Faculty of Philosophy). There is a Metro stop just a few metres from the campus that connects with the centre of the city in 5-10 minutes (Line 3-Facultats). Alternatively, the campus is a 20-25 minute walk from the ‘old town’. Registration Registration will be possible before and during the Symposium at the entrance to the Salon Actos in the Faculty of Philosophy. During the main meeting the registration desk will continue to be available in the Faculty of Philosophy. Oral Presentations All speakers (apart from the symposium speakers) have been allocated 15 minutes. It is therefore expected that you prepare to speak for no more than 12 minutes to allow time for questions and switching between presenters. We have a number of parallel sessions in nearby lecture theatres so timing will be especially important. -

Feeding-Dependent Tentacle Development in the Sea Anemone Nematostella Vectensis ✉ Aissam Ikmi 1,2 , Petrus J
ARTICLE https://doi.org/10.1038/s41467-020-18133-0 OPEN Feeding-dependent tentacle development in the sea anemone Nematostella vectensis ✉ Aissam Ikmi 1,2 , Petrus J. Steenbergen1, Marie Anzo 1, Mason R. McMullen2,3, Anniek Stokkermans1, Lacey R. Ellington2 & Matthew C. Gibson2,4 In cnidarians, axial patterning is not restricted to embryogenesis but continues throughout a prolonged life history filled with unpredictable environmental changes. How this develop- 1234567890():,; mental capacity copes with fluctuations of food availability and whether it recapitulates embryonic mechanisms remain poorly understood. Here we utilize the tentacles of the sea anemone Nematostella vectensis as an experimental paradigm for developmental patterning across distinct life history stages. By analyzing over 1000 growing polyps, we find that tentacle progression is stereotyped and occurs in a feeding-dependent manner. Using a combination of genetic, cellular and molecular approaches, we demonstrate that the crosstalk between Target of Rapamycin (TOR) and Fibroblast growth factor receptor b (Fgfrb) signaling in ring muscles defines tentacle primordia in fed polyps. Interestingly, Fgfrb-dependent polarized growth is observed in polyp but not embryonic tentacle primordia. These findings show an unexpected plasticity of tentacle development, and link post-embryonic body patterning with food availability. 1 Developmental Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany. 2 Stowers Institute for Medical Research, Kansas City, MO 64110, -

Cnidarian Immunity and the Repertoire of Defense Mechanisms in Anthozoans
biology Review Cnidarian Immunity and the Repertoire of Defense Mechanisms in Anthozoans Maria Giovanna Parisi 1,* , Daniela Parrinello 1, Loredana Stabili 2 and Matteo Cammarata 1,* 1 Department of Earth and Marine Sciences, University of Palermo, 90128 Palermo, Italy; [email protected] 2 Department of Biological and Environmental Sciences and Technologies, University of Salento, 73100 Lecce, Italy; [email protected] * Correspondence: [email protected] (M.G.P.); [email protected] (M.C.) Received: 10 August 2020; Accepted: 4 September 2020; Published: 11 September 2020 Abstract: Anthozoa is the most specious class of the phylum Cnidaria that is phylogenetically basal within the Metazoa. It is an interesting group for studying the evolution of mutualisms and immunity, for despite their morphological simplicity, Anthozoans are unexpectedly immunologically complex, with large genomes and gene families similar to those of the Bilateria. Evidence indicates that the Anthozoan innate immune system is not only involved in the disruption of harmful microorganisms, but is also crucial in structuring tissue-associated microbial communities that are essential components of the cnidarian holobiont and useful to the animal’s health for several functions including metabolism, immune defense, development, and behavior. Here, we report on the current state of the art of Anthozoan immunity. Like other invertebrates, Anthozoans possess immune mechanisms based on self/non-self-recognition. Although lacking adaptive immunity, they use a diverse repertoire of immune receptor signaling pathways (PRRs) to recognize a broad array of conserved microorganism-associated molecular patterns (MAMP). The intracellular signaling cascades lead to gene transcription up to endpoints of release of molecules that kill the pathogens, defend the self by maintaining homeostasis, and modulate the wound repair process. -

Life on Our Reefs a Colouring Book
LIFE ON OUR REEFS A COLOURING BOOK Ministry of Fisheries and Agriculture Maté, Republic of Maldives & For Fisheries Development BAY OF BENGAL PROGRAMME This colouring-cum-information book on coral reefs for schoolchildren evolved out of the effort of the subproject ‘Fisheries Extension Services, Maldives (MDV/FES/MDV)’. The focus of the subproject, guided by the fisherfolk, was on building awareness and beginning the consultative processes that would, in time, lead to participatory management of coral reef resources. In doing so, the subproject also reached out to schoolchildren in the islands, the future fisherfolk of the Maldives, through this book. The text, in both Divehi and English, is aimed at primary schoolchildren (6-14 years). The book is intended to be used as a colouring book as well as a reader. It is written by Mr. N.T. Hasen Didi, former Director in the Ministry of Fisheries and Agriculture (MOFA) and an eminent naturalist and artist, and Ms Sana Mohamed, ReefBiologist, Marine Research Section, MOFA. The book was illustrated by Mr. Hussein Zahir, a Biologist on study leave from the Marine Research Section of MOFA, who is also an artist and an enthusiastic diver. The fisheries extension services project, and this book, have been sponsored by the Bay of Bengal Programme’s ‘Small-Scale Fisherfolk Communities in the Bay of Bengal’ (GCP/RAS/ 118/MUL), a project jointly funded by SIDA (Swedish International Development Authority) and DANIDA (Danish International Development Agency) and executed by FAO (Food and Agriculture Organization of the United Nations). The Bay of Bengal Programme (BOBP) is a multiagency regional fisheries programme which covers seven countries around the Bay of Bengal — Bangladesh, India, Indonesia, Malaysia, Maldives, Shri Lanka and Thailand. -
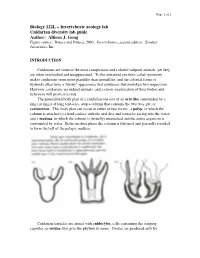
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators. -

The Reproductive Biology of the Scleractinian Coral Plesiastrea Versipora in Sydney Harbour, Australia
Vol. 1: 25–33, 2014 SEXUALITY AND EARLY DEVELOPMENT IN AQUATIC ORGANISMS Published online February 6 doi: 10.3354/sedao00004 Sex Early Dev Aquat Org OPEN ACCESS The reproductive biology of the scleractinian coral Plesiastrea versipora in Sydney Harbour, Australia Alisha Madsen1, Joshua S. Madin1, Chung-Hong Tan2, Andrew H. Baird2,* 1Department of Biological Sciences, Macquarie University, Sydney, New South Wales 2109, Australia 2ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, Queensland 4811, Australia ABSTRACT: The scleractinian coral Plesiastrea versipora occurs throughout most of the Indo- Pacific; however, the species is only abundant in temperate regions, including Sydney Harbour, in New South Wales, Australia, where it can be the dominant sessile organism over small spatial scales. Population genetics indicates that the Sydney Harbour population is highly isolated, sug- gesting long-term persistence will depend upon on the local production of recruits. To determine the potential role of sexual reproduction in population persistence, we examined a number of fea- tures of the reproductive biology of P. versipora for the first time, including the sexual system, the length of the gametogenetic cycles and size-specific fecundity. P. versipora was gonochoric, sup- porting recent molecular work removing the species from the Family Merulinidae, in which the species are exclusively hermaphroditic. The oogenic cycle was between 13 and 14 mo and the spermatogenetic cycle between 7 and 8 mo, with broadcast spawning inferred to occur in either January or February. Colony sex was strongly influenced by colony size: the probability of being male increased with colony area. The longer oogenic cycle suggests that females are investing energy in reproduction rather than growth, and consequently, males are on average larger for a given age. -

The Secret Life of Coral Reefs: a Dominican Republic Adventure TEACHER's GUIDE
The Secret Life of Coral Reefs: A Dominican Republic Adventure TEACHER’S GUIDE Grades: All Subjects: Science and Geography Live event date: May 10th, 2019 at 1:00 PM ET Purpose: This guide contains a set of discussion questions and answers for any grade level, which can be used after the virtual field trip. It also contains links to additional resources and other resources ranging from lessons, activities, videos, demonstrations, experiments, real-time data, and multimedia presentations. Dr. Joseph Pollock Event Description: Coral Strategy Director Is it a rock? Is it a plant? Or is it something else entirely? Caribbean Division The Nature Conservancy Discover the amazing world of coral reefs with coral scientist Joe Pollock, as he takes us on a virtual field trip to the beautiful coastline of the Dominican Republic. We’ll dive into the waters of the Caribbean to see how corals form, the way they grow into reefs, and how they support an incredible array of plants and animals. Covering less than 1% of the ocean floor, coral reefs are home to an estimated 25% of all marine species. That’s why they’re often called the rainforests of the sea! Explore this amazing ecosystem and learn how the reefs are more than just a pretty place—they provide habitat for the fish we eat, compounds for the medicines we take, and even coastal protection during severe weather. Learn how these fragile reefs are being damaged by human activity and climate change, and how scientists from The Nature Conservancy and local organizations are developing ways to restore corals in the areas where they need the most help. -

Biodiversity in the Coral Reefs
Student Name: Class: Biodiversity in the Coral Reefs Expert Pack: Grades 9-10 Table of Contents Text #1: Coral Reefs (Video) ................................................................................................................... 12 Text #2: Top 25 Coral Reef Facts (Informational Text) ................................................................. 13 Text #3: Coral Polyps—Tiny Builders (Scientific Diagram and Informational Text) ........... 18 Text #4: Corals Dine on Microplastics (Informational Text) ...................................................... 20 Text #5: Coral Reef Biodiversity (Informational Text) .................................................................... 24 Text #6: Coral and Coral Reefs (Scientific Article) .......................................................................... 27 Text #7: Bizzare and Beautiful Coral Reef Animals (Website) .................................................... 39 Text #8: The Great Barrier Reef Food Chain (Diagram) ................................................................ 40 Text #9: Why Are Coral Reefs Important (Informational Article) .............................................. 42 Text #10: Status of and Threat to Coral Reefs (Informational Article) .................................... 46 Text #11: Crabs Play Defense, Save Corals (News Article) .......................................................... 54 Text #12: What You Can Do (Informational Article) ...................................................................... 58 Extended Reading, Text -
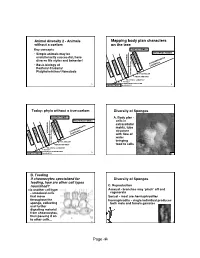
Andivers2'06h.O.Pdf
Animal diversity 2 - Animals Mapping body plan characters without a coelom on the tree Key concepts PROTOSTOMES ARTHROPODA DEUTEROSTOMES NEMATODA • Simple animals may be ANNELIDA MOLLUSCA evolutionarily successful, have PLATYHELMINTHES CNIDARIA RADIAL SYMMETRY diverse life styles and behavior! MOUTH PORIFERA ECHINODERMATA • Basic biology of CHORDATA Porifera/ Cnidaria/ AND Platyhelminthes/ Nematoda ANUS TRUE COELOM MOUTH AND ANUS BILATERAL SYMMETRY TISSUES 1 EXTRACELLULAR DIGESTION 2 PROTISTAMULTICELLULARITY Today: phyla without a true coelom Diversity of Sponges PROTOSTOMES A. Body plan - ARTHROPODA DEUTEROSTOMES NEMATODA cells in ANNELIDA MOLLUSCA extracellular PLATYHELMINTHES CNIDARIA matrix, tube RADIAL SYMMETRY MOUTH PORIFERA ECHINODERMATA structure CHORDATA AND with flow of water ANUS TRUE COELOM bringing MOUTH AND ANUS food to cells BILATERAL SYMMETRY TISSUES EXTRACELLULAR DIGESTION 3 PROTISTAMULTICELLULARITY 4 B. Feeding If choanocytes specialized for Diversity of Sponges feeding, how are other cell types nourished? C. Reproduction via another cell type Asexual - branches may ‘pinch’ off and - amoeboid cells regenerate that move Sexual - most are hermaphrodites throughout the Hermaphrodite - single individual produces sponge, collecting both male and female gametes and further digesting material from choanocytes, then passing it on to other cells... 5 6 Page ‹#› Diversity of Sponges Diversity of Sponges Sperm are produced from modified The zygote (fertilized choanocytes, released egg) gives into the environment rise to swimming -

A Review of Toxins from Cnidaria
marine drugs Review A Review of Toxins from Cnidaria Isabella D’Ambra 1,* and Chiara Lauritano 2 1 Integrative Marine Ecology Department, Stazione Zoologica Anton Dohrn, Villa Comunale, 80121 Napoli, Italy 2 Marine Biotechnology Department, Stazione Zoologica Anton Dohrn, Villa Comunale, 80121 Napoli, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-081-5833201 Received: 4 August 2020; Accepted: 30 September 2020; Published: 6 October 2020 Abstract: Cnidarians have been known since ancient times for the painful stings they induce to humans. The effects of the stings range from skin irritation to cardiotoxicity and can result in death of human beings. The noxious effects of cnidarian venoms have stimulated the definition of their composition and their activity. Despite this interest, only a limited number of compounds extracted from cnidarian venoms have been identified and defined in detail. Venoms extracted from Anthozoa are likely the most studied, while venoms from Cubozoa attract research interests due to their lethal effects on humans. The investigation of cnidarian venoms has benefited in very recent times by the application of omics approaches. In this review, we propose an updated synopsis of the toxins identified in the venoms of the main classes of Cnidaria (Hydrozoa, Scyphozoa, Cubozoa, Staurozoa and Anthozoa). We have attempted to consider most of the available information, including a summary of the most recent results from omics and biotechnological studies, with the aim to define the state of the art in the field and provide a background for future research. Keywords: venom; phospholipase; metalloproteinases; ion channels; transcriptomics; proteomics; biotechnological applications 1.