Regulation of Polyp-To-Jellyfish Transition in Aurelia Aurita
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pupillary Response to Light in Three Species of Cubozoa (Box Jellyfish)
Plankton Benthos Res 15(2): 73–77, 2020 Plankton & Benthos Research © The Plankton Society of Japan Pupillary response to light in three species of Cubozoa (box jellyfish) JAMIE E. SEYMOUR* & EMILY P. O’HARA Australian Institute for Tropical Health and Medicine, James Cook University, 11 McGregor Road, Smithfield, Qld 4878, Australia Received 12 August 2019; Accepted 20 December 2019 Responsible Editor: Ryota Nakajima doi: 10.3800/pbr.15.73 Abstract: Pupillary response under varying conditions of bright light and darkness was compared in three species of Cubozoa with differing ecologies. Maximal and minimal pupil area in relation to total eye area was measured and the rate of change recorded. In Carukia barnesi, the rate of pupil constriction was faster and final constriction greater than in Chironex fleckeri, which itself showed faster and greater constriction than in Chiropsella bronzie. We suggest this allows for differing degrees of visual acuity between the species. We propose that these differences are correlated with variations in the environment which each of these species inhabit, with Ca. barnesi found fishing for larval fish in and around waters of structurally complex coral reefs, Ch. fleckeri regularly found acquiring fish in similarly complex mangrove habitats, while Ch. bronzie spends the majority of its time in the comparably less complex but more turbid environments of shallow sandy beaches where their food source of small shrimps is highly aggregated and less mobile. Key words: box jellyfish, cubozoan, pupillary mobility, vision invertebrates (Douglas et al. 2005, O’Connor et al. 2009), Introduction none exist directly comparing pupillary movement be- The primary function of pupil constriction and dilation tween species in relation to their habitat and lifestyle. -

The Polyp and the Medusa Life on the Move
The Polyp and the Medusa Life on the Move Millions of years ago, unlikely pioneers sparked a revolution. Cnidarians set animal life in motion. So much of what we take for granted today began with Cnidarians. FROM SHAPE OF LIFE The Polyp and the Medusa Life on the Move Take a moment to follow these instructions: Raise your right hand in front of your eyes. Make a fist. Make the peace sign with your first and second fingers. Make a fist again. Open your hand. Read the next paragraph. What you just did was exhibit a trait we associate with all animals, a trait called, quite simply, movement. And not only did you just move your hand, but you moved it after passing the idea of movement through your brain and nerve cells to command the muscles in your hand to obey. To do this, your body needs muscles to move and nerves to transmit and coordinate movement, whether voluntary or involuntary. The bit of business involved in making fists and peace signs is pretty complex behavior, but it pales by comparison with the suites of thought and movement associated with throwing a curve ball, walking, swimming, dancing, breathing, landing an airplane, running down prey, or fleeing a predator. But whether by thought or instinct, you and all animals except sponges have the ability to move and to carry out complex sequences of movement called behavior. In fact, movement is such a basic part of being an animal that we tend to define animalness as having the ability to move and behave. -

Cassiopea Xamachana (Upside-Down Jellyfish)
UWI The Online Guide to the Animals of Trinidad and Tobago Ecology Cassiopea xamachana (Upside-down Jellyfish) Order: Rhizostomeae (Eight-armed Jellyfish) Class: Scyphozoa (Jellyfish) Phylum: Cnidaria (Corals, Sea Anemones and Jellyfish) Fig. 1. Upside-down jellyfish, Cassiopea xamachana. [http://images.fineartamerica.com/images-medium-large/upside-down-jellyfish-cassiopea-sp-pete-oxford.jpg, downloaded 9 March 2016] TRAITS. Cassiopea xamachana, also known as the upside-down jellyfish, is quite large with a dominant medusa (adult jellyfish phase) about 30cm in diameter (Encyclopaedia of Life, 2014), resembling more of a sea anemone than a typical jellyfish. The name is associated with the fact that the umbrella (bell-shaped part) settles on the bottom of the sea floor while its frilly tentacles face upwards (Fig. 1). The saucer-shaped umbrella is relatively flat with a well-defined central depression on the upper surface (exumbrella), the side opposite the tentacles (Berryman, 2016). This depression gives the jellyfish the ability to stick to the bottom of the sea floor while it pulsates gently, via a suction action. There are eight oral arms (tentacles) around the mouth, branched elaborately in four pairs. The most commonly seen colour is a greenish grey-blue, due to the presence of zooxanthellae (algae) embedded in the mesoglea (jelly) of the body, and especially the arms. The mobile medusa stage is dioecious, which means that there are separate males and females, although there are no features which distinguish the sexes. The polyp stage is sessile (fixed to the substrate) and small (Sterrer, 1986). UWI The Online Guide to the Animals of Trinidad and Tobago Ecology DISTRIBUTION. -

PROGRAMME ABSTRACTS AGM Papers
The Palaeontological Association 63rd Annual Meeting 15th–21st December 2019 University of Valencia, Spain PROGRAMME ABSTRACTS AGM papers Palaeontological Association 6 ANNUAL MEETING ANNUAL MEETING Palaeontological Association 1 The Palaeontological Association 63rd Annual Meeting 15th–21st December 2019 University of Valencia The programme and abstracts for the 63rd Annual Meeting of the Palaeontological Association are provided after the following information and summary of the meeting. An easy-to-navigate pocket guide to the Meeting is also available to delegates. Venue The Annual Meeting will take place in the faculties of Philosophy and Philology on the Blasco Ibañez Campus of the University of Valencia. The Symposium will take place in the Salon Actos Manuel Sanchis Guarner in the Faculty of Philology. The main meeting will take place in this and a nearby lecture theatre (Salon Actos, Faculty of Philosophy). There is a Metro stop just a few metres from the campus that connects with the centre of the city in 5-10 minutes (Line 3-Facultats). Alternatively, the campus is a 20-25 minute walk from the ‘old town’. Registration Registration will be possible before and during the Symposium at the entrance to the Salon Actos in the Faculty of Philosophy. During the main meeting the registration desk will continue to be available in the Faculty of Philosophy. Oral Presentations All speakers (apart from the symposium speakers) have been allocated 15 minutes. It is therefore expected that you prepare to speak for no more than 12 minutes to allow time for questions and switching between presenters. We have a number of parallel sessions in nearby lecture theatres so timing will be especially important. -

Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish
diversity Review Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish Michael J. Kingsford * , Jodie A. Schlaefer and Scott J. Morrissey Marine Biology and Aquaculture, College of Science and Engineering and ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD 4811, Australia; [email protected] (J.A.S.); [email protected] (S.J.M.) * Correspondence: [email protected] Abstract: Understanding the hierarchy of populations from the scale of metapopulations to mesopop- ulations and member local populations is fundamental to understanding the population dynamics of any species. Jellyfish by definition are planktonic and it would be assumed that connectivity would be high among local populations, and that populations would minimally vary in both ecological and genetic clade-level differences over broad spatial scales (i.e., hundreds to thousands of km). Although data exists on the connectivity of scyphozoan jellyfish, there are few data on cubozoans. Cubozoans are capable swimmers and have more complex and sophisticated visual abilities than scyphozoans. We predict, therefore, that cubozoans have the potential to have finer spatial scale differences in population structure than their relatives, the scyphozoans. Here we review the data available on the population structures of scyphozoans and what is known about cubozoans. The evidence from realized connectivity and estimates of potential connectivity for scyphozoans indicates the following. Some jellyfish taxa have a large metapopulation and very large stocks (>1000 s of km), while others have clade-level differences on the scale of tens of km. Data on distributions, genetics of medusa and Citation: Kingsford, M.J.; Schlaefer, polyps, statolith shape, elemental chemistry of statoliths and biophysical modelling of connectivity J.A.; Morrissey, S.J. -

Coral Histopathology II
INTRODUCTION The prevalence and severity of reef degradation due to coral disease has increased considerably over the last two decades. Emerging coral diseases have been linked to biotic and abiotic stressors and their synergistic interactions. Coral disease research is in its infancy and only beginning to take advantage of technologies and methodologies routinely used in epizootiology, clinical and diagnostic medicine, and pathology. This Coral Histopathology Workshop (and its predecessor) provided forums to begin adapting advances in biomedical and veterinary sciences, pathology, toxicology, and biotechnology to the study of coral disease and health; it also provided a foundation on which to build a framework for coral disease research that can interface with mainstream medical and veterinary research. The goals of this Workshop were to produce standards for (1) morphological descriptors based on accepted medical terminology, (2) consistent and concise descriptions of lesions in the field, as well as (3) clinical morphological diagnoses in the laboratory. Consensus reached during this workshop will be peer reviewed by the coral scientific community, both in public settings where the materials will be presented in workshop/discussion formats, directly soliciting review by others establishing terminology for the field, and through opinion papers in the peer-review literature to adopt specialized terminologies to facilitate communication among histopathologists. Once adopted, the terminology and microscopic descriptions will enable development of instructional materials and distance learning tools for coral histopathology. STUDY SETS OF HISTOLOGY SLIDES PROVIDED BY THE IRCP An important facet of this workshop was the use of study sets of slides with serial sections from the same sample for independent individual micro- E. -

First Report of the Box Jellyfish Tripedalia Cystophora (Cubozoa
Marine Biodiversity Records, page 1 of 3. # Marine Biological Association of the United Kingdom, 2011 doi:10.1017/S1755267211000133; Vol. 4; e54; 2011 Published online First report of the box jellyfish Tripedalia cystophora (Cubozoa: Tripedaliidae) in the continental USA, from Lake Wyman, Boca Raton, Florida evan r. orellana1 and allen g. collins2 1Gumbo Limbo Nature Centre, 1801 North Ocean Boulevard, Boca Raton, FL 33432, USA, 2NMFS, National Systematics Laboratory, National Museum of Natural History, MRC-153, Smithsonian Institution, PO Box 37012, Washington, DC 20013-7012, USA A male specimen of Tripedalia cystophora (Cubozoa: Tripedaliidae) was collected from Lake Wyman, Boca Raton, Florida, USA. This is the first report of this species from the continental United States and brings the total known number of cubozoan species living in this region to four. Lake Wyman is a natural lagoon/estuary ecosystem which is part of the Atlantic Intracoastal Waterway. The box jellyfish was found in shallow water around the roots of the red mangrove, Rhizophora mangle, where it was observed feeding on copepods attracted to light. This finding may indicate a local population in the waters of south Florida, USA, but an isolated occurrence cannot be ruled out. Keywords: Cubomedusae Submitted 5 May 2010; accepted 21 January 2011 INTRODUCTION MATERIALS AND METHODS Tripedalia cystophora Conant, 1897 is a box jellyfish in the Tripedalia cystophora was collected in Lake Wyman, Boca family Tripedaliidae of the order Carybdeida. Carybdeids Raton, Florida, on 27 September 2009 (26821′59.26′′N are easily identified by the presence of only one tentacle on 80804′16.66′′W). -

Feeding-Dependent Tentacle Development in the Sea Anemone Nematostella Vectensis ✉ Aissam Ikmi 1,2 , Petrus J
ARTICLE https://doi.org/10.1038/s41467-020-18133-0 OPEN Feeding-dependent tentacle development in the sea anemone Nematostella vectensis ✉ Aissam Ikmi 1,2 , Petrus J. Steenbergen1, Marie Anzo 1, Mason R. McMullen2,3, Anniek Stokkermans1, Lacey R. Ellington2 & Matthew C. Gibson2,4 In cnidarians, axial patterning is not restricted to embryogenesis but continues throughout a prolonged life history filled with unpredictable environmental changes. How this develop- 1234567890():,; mental capacity copes with fluctuations of food availability and whether it recapitulates embryonic mechanisms remain poorly understood. Here we utilize the tentacles of the sea anemone Nematostella vectensis as an experimental paradigm for developmental patterning across distinct life history stages. By analyzing over 1000 growing polyps, we find that tentacle progression is stereotyped and occurs in a feeding-dependent manner. Using a combination of genetic, cellular and molecular approaches, we demonstrate that the crosstalk between Target of Rapamycin (TOR) and Fibroblast growth factor receptor b (Fgfrb) signaling in ring muscles defines tentacle primordia in fed polyps. Interestingly, Fgfrb-dependent polarized growth is observed in polyp but not embryonic tentacle primordia. These findings show an unexpected plasticity of tentacle development, and link post-embryonic body patterning with food availability. 1 Developmental Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany. 2 Stowers Institute for Medical Research, Kansas City, MO 64110, -

Cnidarian Immunity and the Repertoire of Defense Mechanisms in Anthozoans
biology Review Cnidarian Immunity and the Repertoire of Defense Mechanisms in Anthozoans Maria Giovanna Parisi 1,* , Daniela Parrinello 1, Loredana Stabili 2 and Matteo Cammarata 1,* 1 Department of Earth and Marine Sciences, University of Palermo, 90128 Palermo, Italy; [email protected] 2 Department of Biological and Environmental Sciences and Technologies, University of Salento, 73100 Lecce, Italy; [email protected] * Correspondence: [email protected] (M.G.P.); [email protected] (M.C.) Received: 10 August 2020; Accepted: 4 September 2020; Published: 11 September 2020 Abstract: Anthozoa is the most specious class of the phylum Cnidaria that is phylogenetically basal within the Metazoa. It is an interesting group for studying the evolution of mutualisms and immunity, for despite their morphological simplicity, Anthozoans are unexpectedly immunologically complex, with large genomes and gene families similar to those of the Bilateria. Evidence indicates that the Anthozoan innate immune system is not only involved in the disruption of harmful microorganisms, but is also crucial in structuring tissue-associated microbial communities that are essential components of the cnidarian holobiont and useful to the animal’s health for several functions including metabolism, immune defense, development, and behavior. Here, we report on the current state of the art of Anthozoan immunity. Like other invertebrates, Anthozoans possess immune mechanisms based on self/non-self-recognition. Although lacking adaptive immunity, they use a diverse repertoire of immune receptor signaling pathways (PRRs) to recognize a broad array of conserved microorganism-associated molecular patterns (MAMP). The intracellular signaling cascades lead to gene transcription up to endpoints of release of molecules that kill the pathogens, defend the self by maintaining homeostasis, and modulate the wound repair process. -

Life on Our Reefs a Colouring Book
LIFE ON OUR REEFS A COLOURING BOOK Ministry of Fisheries and Agriculture Maté, Republic of Maldives & For Fisheries Development BAY OF BENGAL PROGRAMME This colouring-cum-information book on coral reefs for schoolchildren evolved out of the effort of the subproject ‘Fisheries Extension Services, Maldives (MDV/FES/MDV)’. The focus of the subproject, guided by the fisherfolk, was on building awareness and beginning the consultative processes that would, in time, lead to participatory management of coral reef resources. In doing so, the subproject also reached out to schoolchildren in the islands, the future fisherfolk of the Maldives, through this book. The text, in both Divehi and English, is aimed at primary schoolchildren (6-14 years). The book is intended to be used as a colouring book as well as a reader. It is written by Mr. N.T. Hasen Didi, former Director in the Ministry of Fisheries and Agriculture (MOFA) and an eminent naturalist and artist, and Ms Sana Mohamed, ReefBiologist, Marine Research Section, MOFA. The book was illustrated by Mr. Hussein Zahir, a Biologist on study leave from the Marine Research Section of MOFA, who is also an artist and an enthusiastic diver. The fisheries extension services project, and this book, have been sponsored by the Bay of Bengal Programme’s ‘Small-Scale Fisherfolk Communities in the Bay of Bengal’ (GCP/RAS/ 118/MUL), a project jointly funded by SIDA (Swedish International Development Authority) and DANIDA (Danish International Development Agency) and executed by FAO (Food and Agriculture Organization of the United Nations). The Bay of Bengal Programme (BOBP) is a multiagency regional fisheries programme which covers seven countries around the Bay of Bengal — Bangladesh, India, Indonesia, Malaysia, Maldives, Shri Lanka and Thailand. -
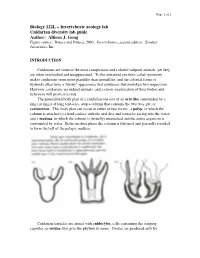
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators. -

The Reproductive Biology of the Scleractinian Coral Plesiastrea Versipora in Sydney Harbour, Australia
Vol. 1: 25–33, 2014 SEXUALITY AND EARLY DEVELOPMENT IN AQUATIC ORGANISMS Published online February 6 doi: 10.3354/sedao00004 Sex Early Dev Aquat Org OPEN ACCESS The reproductive biology of the scleractinian coral Plesiastrea versipora in Sydney Harbour, Australia Alisha Madsen1, Joshua S. Madin1, Chung-Hong Tan2, Andrew H. Baird2,* 1Department of Biological Sciences, Macquarie University, Sydney, New South Wales 2109, Australia 2ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, Queensland 4811, Australia ABSTRACT: The scleractinian coral Plesiastrea versipora occurs throughout most of the Indo- Pacific; however, the species is only abundant in temperate regions, including Sydney Harbour, in New South Wales, Australia, where it can be the dominant sessile organism over small spatial scales. Population genetics indicates that the Sydney Harbour population is highly isolated, sug- gesting long-term persistence will depend upon on the local production of recruits. To determine the potential role of sexual reproduction in population persistence, we examined a number of fea- tures of the reproductive biology of P. versipora for the first time, including the sexual system, the length of the gametogenetic cycles and size-specific fecundity. P. versipora was gonochoric, sup- porting recent molecular work removing the species from the Family Merulinidae, in which the species are exclusively hermaphroditic. The oogenic cycle was between 13 and 14 mo and the spermatogenetic cycle between 7 and 8 mo, with broadcast spawning inferred to occur in either January or February. Colony sex was strongly influenced by colony size: the probability of being male increased with colony area. The longer oogenic cycle suggests that females are investing energy in reproduction rather than growth, and consequently, males are on average larger for a given age.