Mandated Lowering of Toxicants in Cigarette Smoke
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Pyramid Cigarettes
** Pyramid Cigarettes ** Pyramid Red Box 10 Carton Pyramid Blue Box 10 Carton Pyramid Menthol Gold Box 10 Carton Pyramid Menthol Silver Box 10 Carton Pyramid Orange Box 10 Carton Pyramid Red Box 100 10 Carton Pyramid Blue Box 100 10 Carton Pyramid Menthol Gold Box 100 10 Carton Pyramid Menthol Silver Box 100 10 Carton Pyramid Orange Box 100 10 Carton Pyramid Non Filter Box 10 Carton ** E Cigarettes ** Logic Disposable E Cigarette Menthol Gold 24 Box Logic Disposable E Cigarette Menthol High 24 Box Logic Disposable E Cigarette Menthol Platinum 24 Box Logic Disposable E Cigarette Menthol Sterling 24 Box Logic Disposable E Cigarette Menthol Zero 24 Box Logic Disposable E Cigarette Gold 24 Box Logic Disposable E Cigarette High 24 Box Logic Disposable E Cigarette Sterling 24 Box Logic Disposable E Cigarette Platinum 24 Box Logic Disposable E Cigarette Zero 24 Box ** Premium Cigars ** Acid Krush Classic Blue 5-10pk Tin Acid Krush Classic Mad Morado 5-10pk Tin Acid Krush Classic Gold 5-10pk Tin Acid Krush Classic Red 5-10pk Tin Acid Kuba Kuba 24 Box Acid Blondie 40 Box Acid C-Note 20 Box Acid Kuba Maduro 24 Box Acid 1400cc 18 Box Acid Blondie Belicoso 24 Box Acid Kuba Deluxe 10 Box Acid Cold Infusion 24 Box Ambrosia Clove Tiki 10 Box Acid Larry 10-3pk Pack Acid Deep Dish 24 Box Acid Wafe 28 Box Acid Atom Maduro 24 Box Acid Nasty 24 Box Acid Roam 10 Box Antano Dark Corojo Azarosa 20 Box Antano Dark Corojo El Martillo 20 Box Antano Dark Corojo Pesadilla 20 Box Antano Dark Corojo Poderoso 20 Box Natural Dirt 24 Box Acid Liquid 24 Box Acid Blondie -

Order Catalog - Spring 2015
760 S. Delsea Drive, PO Box 1447 Vineland, New Jersey 08362-1447 Visit us on the web at: www.ljzucca.com PHONE 856-692-7425 TOLL FREE # 1-800-552-2639 FAX # 800-443-2067 ORDER CATALOG - SPRING 2015 Orders MUST be placed by 3:00 p.m. the day before your scheduled delivery day Customer Number Customer Name Day of delivery Street Address Date of delivery City Taken by ITEM# QTY DESCRIPTION ****ONLINE ORDERING NOW AVAILABLE FOR DELIVERED ORDERS**** PLEASE CONTACT CUSTOMER SERVICE To order catalogs use item number 803205 1-800-934-3968 www.wecard.org Office Hours: 7:00 am - 4:00 pm Monday - Friday Showroom Hours: 8:30 am - noon, 1:00 pm - 4:30 pm Monday - Thursday 8:30 am - noon, 1:00 pm - 3:00 pm Friday RETURN POLICY If an item is received with LESS than the guaranteed shelf life and you believe you will not sell it before the expiration date: Contact our office within 72 hours with the invoice number, item number, quantity, and the code on the received item. The following items may be returned for credit ONLY IF PURCHASED FROM L J ZUCCA in the last 14 days and in original packaging, un-opened, and never stickered. BATTERIES GROCERIES (Case) and (Each) CANDY, GUM, MINTS FULL BOXES PAPER PRODUCTS CIGARETTE PAPERS PLAYING CARDS CIGARETTE TUBES PIPES GLOVES SPORT & TRADING CARDS Credit for the following product categories is divided into four sections FULL CREDIT RESTRICTIONS ANY "NEW" CANDY, NOVELTY, GUM, SNACK ITEM Within 60 days of our initial release CIGARS PACKS KETTLE CHIPS MEAT SNACKS NABISCO CRACKERS & COOKIES TOBACCO CREDIT WITH RESTRICTIONS RESTRICTIONS: BEVERAGE'S If received with LESS than 30 days shelf life CANDY, GUM, CRACKERS OR COOKIES If received with LESS than 30 days shelf life CANDY - HOLIDAY If returned 30 days BEFORE the Holiday CIGAR FULL BOXES Full Boxes, original packaging, un-opened, and never stickered. -

Warn About the Dangers of Tobacco
Building Capacity for Tobacco Control / Training Package 2 Warn about the dangers of tobacco: Packaging and labelling of tobacco products Warn about the dangers of tobacco: Packaging and labelling of tobacco products and labelling of tobacco Packaging of tobacco: about the dangers Warn WHO Library Cataloguing-in-Publication Data Building capacity for tobacco control: training package. Contents: 1.Protect people from tobacco smoke: smoke-free environments - 2.Warn about the dangers of tobacco: packaging and labelling of tobacco products - 3.Tobacco advertising, promotion and sponsorship: enforcing comprehensive bans. 1.Tobacco smoke pollution - prevention and control. 2.Smoking - prevention and control. 3.Tobacco-derived products labelling. 4.Tobacco control campaigns. 5.Teaching materials I.World Health Organization. II.International Union against Tuberculosis and Lung Disease. ISBN 978 92 4 150135 4 (NLM classification: HD 9130.6) © World Health Organization and International Union against Tuberculosis and Lung Disease 2011 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Cigarette Mainstream Smoke: the Evolution of Methods and Devices for Generation, Exposure and Collection * By
Beiträge zur Tabakforschung International Contributions to Tobacco Research Volume 27 @ No. 4 @ October 2016 DOI: 10.1515/cttr-2016-0015 Cigarette Mainstream Smoke: The Evolution of Methods and Devices for Generation, Exposure and Collection * by Hubert Klus 1, Barbara Boenke-Nimphius 2, and Lutz Müller 3 1 Oriongasse 9,3100 St. Pölten, Austria 2 Beiträge zur Tabakforschung International, Chausseestraße 51A, 10115 Berlin, Germany 3 Stralsunder Straße 1, 01109 Dresden, Germany SUMMARY (filters and traps) developed over time - some for very specific purposes - and refers to the perpetual problem of The objective of this review is to support tobacco scientists artifact formation by aging. [Beitr. Tabakforsch. Int. 27 when evaluating information published on smoking ma- (2016) 137–274] chines, and on cigarette mainstream smoke (in vivo and in vitro) exposure systems and collection devices. The intriguing development of smoking machines (mainly ZUSAMMENFASSUNG for cigarettes) is followed for more than 170 years - from the first simple set-ups in the 1840s to the sophisticated and Es ist die Intention dieser Übersicht, auf dem Gebiet des fully automated analytical smoking machines available Tabaks arbeitende Wissenschaftler zu unterstützen bei der today. Systems for the large-scale production of smoke Bewertung publizierter Informationen über Rauchmaschi- (condensate) for preparative work are equally considered. nen sowie über Systeme zur experimentellen Exposition (in The standardization of machine smoking methods and test vivo und in vitro) mit Zigarettenhauptstromrauch und pieces has solved several technical problems and produced Apparate zu dessen Sammlung. sensible rules but, at the same time, given rise to new Die sehr interessante Entwicklung von Rauchmaschinen controversies like the compatibility of artificial and human (vornehmlich für Zigaretten) wird über einen Zeitraum von smoking, and the implementation of more intense machine mehr als 170 Jahren nachgezeichnet - von den ersten ein- smoking regimes. -

Motivations for Competitor 'Lights' Imms. Marlboro Lights
. Imms ke Mi MARKETING PLANNING & RESEARCH Motivations for Competitor 'Lights' Marlboro Lights Report of Qualitative Research Study Prepared for : Gallaher Limited February 1996 Mike Imms, Hope Cottage, School Hill, Slindon, Nr. Arundel, West Sussex BN18 ORA Tel: 01243 814777 Fax : 01243 814700 VAT Registration No 5'_S 3541 43 Contents i) Background and objectives of the research ii) Details of the research - sampling issues, sample and stimulus material iii) Management Summary of Main Findings Main Findings 1 . Context - perceptions of Silk Cut and low tar 2. Marlboro Lights as a product 3. Marlboro Lights and it's smoker base 4. Marlboro Lights as a brand 5. Marlboro Lights marketing - packaging, promotions and advertising 6. Conclusions and implications for Silk Cut NOTE : This report should be seen in conjunction with another separate project carried out in tandem exploring Motivations for Lambert & Butler Lights i) Background and objectives of the research This project sought to understand smoker motivations for Marlboro Lights and was one of two projects carried out separately but in tandem exploring issues which affect Silk Cut. The other, 'tandem' project explored smoker motivations for another quite different low tar brand - Lambert and Butler Lights . Over the past twelve months, both Marlboro Lights and L & B Lights have shown significant share growth, and this has given rise to three marketing concerns a) whilst much of the growth of both brands has come from the 'parent' full strength variants, duplication data shows that 'most often' smokers of both brands are also more likely to duplicate with Silk Cut than any other brand . -

Seatca Packaging Design (25Feb2020)Web
No logos, colours, Pictorial health brand images or warnings used in promotional conjunction with information standardised packaging SMOKING CAUSES LUNG CANCER Pack surfaces in a standard colour Brand and product names in a standard colour and font 2020 Southeast Asia Tobacco Control Alliance Packaging Design Analysis to Support Standardised Packaging in the ASEAN Authors: Tan Yen Lian and Yong Check Yoon Editorial Team: Southeast Asia Tobacco Control Alliance Suggested citation: Tan YL. and Yong CY. (2020). Packaging Design Analysis to Support Standardised Packaging in the ASEAN, January 2020. Southeast Asia Tobacco Control Alliance (SEATCA), Bangkok. Thailand. Published by: Southeast Asia Tobacco Control Alliance (SEATCA) Thakolsuk Place, Room 2B, 115 Thoddamri Road, Dusit, Bangkok 10300 Thailand Telefax: +66 2 241 0082 Acknowledgment We would like to express our sincere gratitude to our country partners for their help in purchasing the cigarette packs from each country for the purpose of the study, which contributed to the development of this report. Disclaimer The information, ndings, interpretations, and conclusions expressed herein are those of the author(s) and do not necessarily reect the views of the funding organization, its sta, or its Board of Directors. While reasonable eorts have been made to ensure the accuracy of the information presented at the time of publication, SEATCA does not guarantee the completeness and accuracy of the information in this document and shall not be liable for any damages incurred as a result of its use. Any factual errors or omissions are unintentional. For any corrections, please contact SEATCA at [email protected]. © Southeast Asia Tobacco Control Alliance 2020 This document is the intellectual property of SEATCA and its authors. -

Commercial Tobacco Free Policy Guide
Commercial Tobacco Free Policy Guide Developed with funding from Centers for Disease Control and Prevention: A Comprehensive Approach to Good Health and Wellness in Indian Country. Grant #6NU58DP005432-02-01 Administered by the California Rural Indian Health Board, Inc. This page left intentionally blank 2 Commercial Free Policy Resource Guide Table of Contents Section 1: The History and Role of Tobacco Among American 4 Indians in California Traditional Tobacco 4 Commercial Tobacco 5 Commercial Tobacco Related Health Disparities 6 Commercial Secondhand Smoke and Its Impact 8 Exposure to Commercial Secondhand Smoke 9 Section 2: Commercial Tobacco and Policy Why a Commercial Smoke Free Policy 11 Types of Commercial Smoke Free Policies 12 Developing Support for and Adoption of Commercial Smoke Free Policies 15 Common Commercial Smoke Free Talking Points 16 Special Considerations for Writing a Commercial Smoke Free Policy 16 Presenting Policy to Tribal Council 17 Implementation of Commercial Smoke Free Policies 19 Policy Review Checklist 20 Tribal Policy Examples 22 Tribal Policy Highlights 24 Section 3: Assist Patients to Quit 29 The 5 A’s for Clinicians 30 The 2 A’s and R for Community Health Representatives and Social Workers 31 Tribal Cessation Resources 32 3 Section 1: The History and Role of Tobacco Among American Indians in California Traditional Tobacco American Indians in California have a long history with tobacco. This history dates over thousands of years. For some Tribes, tobacco was a gift from the creator, and given to the people at the time of creation or in times of need. The Yokuts of Central California believed that tobacco was chewed by Hawk Spirit, and after becoming wise from chewing the tobacco, Hawk Spirit created the mountains. -

Plain Packaging of Tobacco Products - a Case Study in Radical Health Policy Adoption and the Role of Advocacy
2013 Cunningham Lecture: Plain packaging of tobacco products - A case study in radical health policy adoption and the role of advocacy Simon Chapman On 1 December 2012 Australia became the first country to implement plain packaging of tobacco products. The legislation was unopposed by the Opposition and supported by the Greens and all independents except Bob Katter. No nation has ever mandated the entire appearance of packaging for any consumer product. So this historic legislation was at once exceptional and radical. It was also a massive threat to the transnational tobacco industry which quickly unleashed an unprecedented global campaign to try to defeat the Bill and deter others from following Australia. Across a 35-year career in tobacco control, I’ve never seen anything like it. The tobacco industry spent well over $14 million on TV and other media campaigns. It was humiliated in a failed 6-1 High Court challenge. It encouraged corrupt and several puppet governments with zero tobacco trade with Australia to launch complaints in the World Trade Organisation. It funded folksy astro-turf retail groups, who dutifully screamed in high dudgeon about the catastrophic job losses that would occur when a policy that was repeatedly predicted to ‘not work’, actually would do what it was meant to do and reduce tobacco sales. And it donated to the Institute of Public Affairs where the resident errand boys dutifully telegraphed their masters’ voices about how the nanny state was now running amok. In tobacco control we call these reactions ‘the scream test’ of policy potency. We’re amused when the industry supports anodyne, useless campaigns like these. -
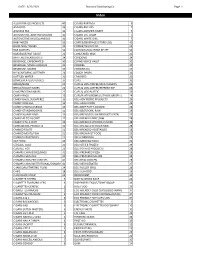
3/22/2021 Standard Distributing Co. Page 1 ALLIGATOR ICE
DATE: 3/22/2021 Standard Distributing Co. Page 1 Index ALLIGATOR ICE PRODUCTS 40 CIGARS-PARTAGA 6 ANTACIDS 33 CIGARS-PHILLIES 7 ARIZONA TEA 31 CIGARS-SWISHER SWEET 7 AUTOMOTIVE-ADDITIVES/FLUIDS 35 CIGARS-U.S. CIGAR 7 AUTOMOTIVE-MISCELLANEOUS 36 CIGARS-WHITE OWL 7 BABY NEEDS 33 COFFEE/BEVERAGE (STORE USE) 40 BAND AIDS/ SWABS 33 COFFEE/TEA/COCOA 23 BAR SUPPLIES 34 COLD/SINUS/ALLERGY RELIEF 33 BARBEQUE/HOT SAUCE 22 CONDENSED MILK 23 BATTERIES/FLASHLIGHTS 34 CONDOMS 34 BEVERAGE, CARBONATED 30 CONVENIENCE VALET 32 BEVERAGE, MISCELLANEOUS 31 COOKIES 20 BEVERAGE, MIXERS 30 COOKING OIL 23 BLEACH/FABRIC SOFTENER 24 COUGH DROPS 33 BOTTLED WATER 31 CRACKERS 20 BOWLS/PLATES/UTENSILS 37 CUPS 36 BREAD/BUNS 27 CUPS & LIDS-COFFEE/JAVA CLASSICS 36 BREAD/DOUGH MIXES 22 CUPS & LIDS-COFFEE/REFRESH EXP 36 CAKE/FROSTING MIXES 22 CUPS & LIDS-PLASTIC 36 CANDY-BAGS 15 CUPS/PLATES/BOWLS/UTENSILS(RESELL) 24 CANDY-BAGS, SUGARFREE 16 DELI-ADV PIERRE PRODUCTS 29 CANDY-KING SIZE 12 DELI-ASIAN FOOD 29 CANDY-MISCELLANEOUS 13 DELI-BEEF PATTY,COOKED 28 CANDY-STANDARD BARS 11 DELI-BEEF/PORK, RAW 28 CANDY-XLARGE BARS 13 DELI-BREAD/DOUGH PRODUCTS FRZN 29 CANDY-45 TO 10 CENT 12 DELI-BREADED BEEF,RAW 28 CANDY-5 TO 2 CENT 12 DELI-BREADED CHICKEN,COOKED 28 CANNABIDIOL PRODUCTS 32 DELI-BREADED CHICKEN,RAW 28 CANNED FRUITS 21 DELI-BREADED VEGETABLES 28 CANNED MEATS/FISH 21 DELI-BREAKFAST FOOD 29 CANNED VEGETABLES 21 DELI-CORNDOGS 29 CAT FOOD 23 DELI-MEXICAN FOOD 29 CEREALS, COLD 23 DELI-OTHER FROZEN 29 CEREALS, HOT 23 DELI-POTATO PRODUCTS 28 CHAMPS CHKN BOXES/BAGS 41 DELI-PREPARED -

WHO Study Group on Tobacco Product Regulation
WHO Technical Report Series 1001 WHO study group on tobacco product regulation Report on the scientific basis of tobacco product regulation: Sixth report of a WHO study group The World Health Organization was established in 1948 as a specialized agency of the United Nations serving as the directing and coordinating authority for international health matters and public health. One of WHO’s constitutional functions is to provide objective and reliable information and advice in the field of human health, a responsibility that it fulfils in part through its extensive programme of publications. The Organization seeks through its publications to support national health strategies and address the most pressing public health concerns of populations around the world. To respond to the needs of Member States at all levels of development, WHO publishes practical manuals, handbooks and training material for specific categories of health workers; internationally applicable guidelines and standards; reviews and analyses of health policies, programmes and research; and state-of-the-art consensus reports that offer technical advice and recommendations for decision-makers. These books are closely tied to the Organization’s priority activities, encompassing disease prevention and control, the development of equitable health systems based on primary health care, and health promotion for individuals and communities. Progress towards better health for all also demands the global dissemination and exchange of information that draws on the knowledge and experience of all WHO’s Member countries and the collaboration of world leaders in public health and the biomedical sciences. To ensure the widest possible availability of authoritative information and guidance on health matters, WHO secures the broad international distribution of its publications and encourages their translation and adaptation. -

Tobacco in Australia Facts & Issues
Tobacco in Australia Facts & Issues A comprehensive online resource tobaccoinaustralia.org.au Book excerpt List of chapters available at tobaccoinaustralia.org.au Introduction Chapter 1 Trends in the prevalence of smoking Chapter 2 Trends in tobacco consumption Chapter 3 The health effects of active smoking Chapter 4 The health effects of secondhand smoke Chapter 5 Factors influencing the uptake and prevention of smoking Chapter 6 Addiction Chapter 7 Smoking cessation Chapter 8 Tobacco use among Aboriginal peoples and Torres Strait Islanders Chapter 9 Smoking and social disadvantage Chapter 10 The tobacco industry in Australian society Chapter 11 Tobacco advertising and promotion Chapter 12 The construction and labelling of Australian cigarettes Chapter 13 The pricing and taxation of tobacco products in Australia Chapter 14 Social marketing and public education campaigns Chapter 15 Smokefree environments Chapter 16 Tobacco litigation in Australia Chapter 17 The economics of tobacco control Chapter 18 The WHO Framework Convention on Tobacco Control Appendix 1 Useful weblinks to tobacco resources Tobacco in Australia: Facts and Issues. Fourth Edition A comprehensive review of the major issues in smoking and health in Australia, compiled by Cancer Council Victoria. First edition published by ASH (Australia) Limited, Surry Hills, NSW, 1989 Second edition published by the Victorian Smoking and Health Program, Carlton South, Victoria (Quit Victoria), 1995 Third edition published by Cancer Council Victoria 2008 in electronic format only. ISBN number: 978-0-947283-76-6 Suggested citation: Scollo, MM and Winstanley, MH. Tobacco in Australia: Facts and issues. 4th edn. Melbourne: Cancer Council Victoria; 2012. Available from www.TobaccoInAustralia.org.au OR <Author(s) of relevant chapter section>, <Name of chapter section> in Scollo, MM and Winstanley, MH [editors]. -

"Sold American!" the First Fifty Years
Digitized by the Internet Arcliive in 2014 https://archive.org/details/soldamericanfirsOOamer 1st MAY^ t9\C. CONTENTS FOREWORD 5 THE MAKINGS OF A NATION 1492-1864 7 PRO BONO PUBLICO 1865- 1903 17 OUT OF MANY, ONE 1904- 1911 35 ASK DAD, HE KNOWS 1912-1925 45 "WHAT THIS COUNTRY NEEDS >> 59 NATURE IN THE RAW 1926- 1938 73 MODERN DESIGN 1939- 1946 87 L.S./M.F.T. 1947- 1949 99 PARTICULAR PEOPLE 1950-1954 113 ! SOLD AMERICAN — THE FIRST FIFTY YEARS COPYRIGHT, 1954 THE AMERICAN TOBACCO COMPANY "SOLD AMERICAN!" PRESENTS A PICTURE OF THE EVOLUTION OF THE COMPANY, REFLECTING CHIEFLY EVENTS OF SUCCESSIVE PERIODS OF CHANGE DURING THE LAST FIFTY YEARS, AS RE- COUNTED BY THE AUTHOR. THE AUTHOR HAS DRAWN UPON LEGENDS AS WELL AS HISTORICALLY ESTABLISHED FACTS AND UPON THE COMPANY'S RECORDS AND THE PERSONAL RECOLLECTIONS OF ITS PRESENT-DAY OFFICIALS, PERSONNEL AND ASSOCIATES. IT IS NOT INTENDED AS A STATEMENT OF COMPANY POLICY AT ANY GIVEN TIME. Powhatan, symbol of American Tobacco, was a chief of Virginia Indians. His daughter Pocahontas wed John Rolfe, the first white man of the Jamestown settlement to grow a commercial crop of tobacco. 4 FOREWORD ON OCTOBER 19, 1904, The American Tobacco fresh uses of the printed word. But by these tokens Company in its present legal form came into it is also true that our Company has had more experi- being. In a legal sense, then, this history marks our ence in cigarette making, more opportunity to learn fiftieth anniversary. our business, than any other. Like many other statistics, this one tells a very This means that we have managed to plow incomplete story.