WHO Study Group on Tobacco Product Regulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

“They Twisted My Arm to Get These Deals.”
03-04_TAA_catalog.cg3 7/1/03 12:35 PM Page 1 2003/2004 2003 PIPE OF THE YEAR Erik says: AT PRICES “They twisted my arm “YOU WILL NEVER SEE AGAIN!” to get these deals.” SEE PAGE 2 03-04_TAA_catalog.cg3 7/1/03 12:35 PM Page 2 “They twisted my arm to get these No two handmade pipes The best value deals, you can’t beat these prices!” are exactly alike. on the market! EXCLUSIVE Bjarne Nielsen offers an The famous Danish pipe maker, Erik Nording, excellent pipe at a fair price. presents OCASO pipes at prices you His reputation depends on it. will never see again! Bjarne churchwarden pipes 3C 2003 Pipe of the Year are the latest rage, but all of these shapes are exquisite. 3D* 2B 3E 2C * 3F 2A 2A Nording Freehand Tall, smooth. 2C Nording Freehand Apple, with carving. 3G* Normally $200. Now only $110.00 Normally $100. Now only $59.95 2B Nording Freehand Tall, with carving. 2D Nording Freehand Apple, smooth. *FLAT Normally $100. Now only $59.95 Normally $200. Now only $110 (not shown) BOTTOM “SITTERS” 3A 3B 3H* TABLE OF CONTENTS Cigars . 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 bjarne Pipes . 31, 32, 33, 35, 36, 37, 38, 40, 40, 41, 48, 50 Cigarettes and Accessories . 16, 42, 43, 44, 46, 47, 48 3A Churchwarden with Carving. $65 Flasks and Barware . 49 3B Churchwarden Smooth. $79 Humidors . 10, 11 3C Bent Prince with Carving. $65 Lighters. 15, 17, 39, 51 3D Freehand Sitter with Carving. -

Large Firm Dynamics and Secular Stagnation: Evidence from Japan and the U.S
Bank of Japan Working Paper Series Large Firm Dynamics and Secular Stagnation: Evidence from Japan and the U.S. Yoshihiko Hogen* [email protected] Ko Miura** [email protected] Koji Takahashi*** [email protected] No.17-E-8 Bank of Japan June 2017 2-1-1 Nihonbashi-Hongokucho, Chuo-ku, Tokyo 103-0021, Japan ***Research and Statistics Department (currently at the Monetary Affairs Department) *** Research and Statistics Department *** Research and Statistics Department (currently at the Financial System and Bank Examination Department) Papers in the Bank of Japan Working Paper Series are circulated in order to stimulate discussion and comments. Views expressed are those of authors and do not necessarily reflect those of the Bank. If you have any comment or question on the working paper series, please contact each author. When making a copy or reproduction of the content for commercial purposes, please contact the Public Relations Department ([email protected]) at the Bank in advance to request permission. When making a copy or reproduction, the source, Bank of Japan Working Paper Series, should explicitly be credited. Large Firm Dynamics and Secular Stagnation: Evidence from Japan and the U.S. Yoshihiko Hogeny Ko Miuraz Koji Takahashix June 2017 Abstract Focusing on the recent secular stagnation debate, this paper examines the role of large …rm dynamics as determinants of productivity ‡uctuations. We …rst show that idiosyncratic shocks to large …rms as well as entry, exit, and reallocation e¤ects account for 30 to 40 percent of productivity ‡uctuations in Japan and the U.S. -

Supplementary Appendix
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Brown JE, Luo W, Isabelle LM, Pankow JF. Candy flavorings in tobacco. N Engl J Med 2014;370: 2250-2. DOI: 10.1056/NEJMc1403015 N ENGL J MED NEJM.ORG Supplementary Appendix ‐ 2014 Candy Flavorings in Tobacco: Brown, Luo, Isabelle, Pankow Candy Flavorings in Tobacco Jessica E. Brown, B.S. Wentai Luo, M.S., Ph.D. Lorne M. Isabelle, M.S. James F. Pankow, M.S., Ph.D. From: Portland State University, Department of Civil & Environmental Engineering (J.E.B., L.M.I., W.L., J.F.P.); Portland State University, Department of Chemistry, (L.E.M., W.L, J.F.P.). Address reprint requests to Dr. Pankow at Department of Civil & Environmental Engineering, Portland State University, Portland, Oregon, 97207-0751; [email protected]. Table of Contents page flavors include “green apple” and “strawberry”. Flavored Tobacco Products in the World S-1 Sutra cigarettes (C.H. Graphics, India) are avail- Cigarettes vs. “Cigars” S-2 able in “grape”, “cherry”, and “vanilla”. Sweet Selling Tobacco to Youth S-2 Methods: Analyses of Candies and Kool-Aid S-3 Dreams cigarettes (Donskoy Tabak JSC, Russia) Methods: Analyses of Tobacco Products S-3 are available in a vanilla/cherry/chocolate mix, Results S-4 and in “double apple”. Kiss cigarettes (Donskoy References S-6 Tabak JSC) are available in “strawberry” and Table S1. List of 70 Flavor Chemicals S-8 Table S2. Compounds in “Cherry” Products S-16 “fresh apple”. Black Devil cigarettes (Huepink & Table S3. -
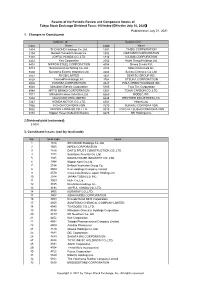
Published on July 21, 2021 1. Changes in Constituents 2
Results of the Periodic Review and Component Stocks of Tokyo Stock Exchange Dividend Focus 100 Index (Effective July 30, 2021) Published on July 21, 2021 1. Changes in Constituents Addition(18) Deletion(18) CodeName Code Name 1414SHO-BOND Holdings Co.,Ltd. 1801 TAISEI CORPORATION 2154BeNext-Yumeshin Group Co. 1802 OBAYASHI CORPORATION 3191JOYFUL HONDA CO.,LTD. 1812 KAJIMA CORPORATION 4452Kao Corporation 2502 Asahi Group Holdings,Ltd. 5401NIPPON STEEL CORPORATION 4004 Showa Denko K.K. 5713Sumitomo Metal Mining Co.,Ltd. 4183 Mitsui Chemicals,Inc. 5802Sumitomo Electric Industries,Ltd. 4204 Sekisui Chemical Co.,Ltd. 5851RYOBI LIMITED 4324 DENTSU GROUP INC. 6028TechnoPro Holdings,Inc. 4768 OTSUKA CORPORATION 6502TOSHIBA CORPORATION 4927 POLA ORBIS HOLDINGS INC. 6503Mitsubishi Electric Corporation 5105 Toyo Tire Corporation 6988NITTO DENKO CORPORATION 5301 TOKAI CARBON CO.,LTD. 7011Mitsubishi Heavy Industries,Ltd. 6269 MODEC,INC. 7202ISUZU MOTORS LIMITED 6448 BROTHER INDUSTRIES,LTD. 7267HONDA MOTOR CO.,LTD. 6501 Hitachi,Ltd. 7956PIGEON CORPORATION 7270 SUBARU CORPORATION 9062NIPPON EXPRESS CO.,LTD. 8015 TOYOTA TSUSHO CORPORATION 9101Nippon Yusen Kabushiki Kaisha 8473 SBI Holdings,Inc. 2.Dividend yield (estimated) 3.50% 3. Constituent Issues (sort by local code) No. local code name 1 1414 SHO-BOND Holdings Co.,Ltd. 2 1605 INPEX CORPORATION 3 1878 DAITO TRUST CONSTRUCTION CO.,LTD. 4 1911 Sumitomo Forestry Co.,Ltd. 5 1925 DAIWA HOUSE INDUSTRY CO.,LTD. 6 1954 Nippon Koei Co.,Ltd. 7 2154 BeNext-Yumeshin Group Co. 8 2503 Kirin Holdings Company,Limited 9 2579 Coca-Cola Bottlers Japan Holdings Inc. 10 2914 JAPAN TOBACCO INC. 11 3003 Hulic Co.,Ltd. 12 3105 Nisshinbo Holdings Inc. 13 3191 JOYFUL HONDA CO.,LTD. -

Other Tobacco Products (OTP) Are Products Including Smokeless and “Non-Cigarette” Materials
Other tobacco products (OTP) are products including smokeless and “non-cigarette” materials. For more information on smoking and how to quit using tobacco products, check out our page on tobacco. A tobacco user may actually absorb more nicotine from chewing tobacco or snuff than they do from a cigarette (Mayo Clinic). The health consequences of smokeless tobacco use include oral, throat and pancreatic cancer, tooth loss, gum disease and increased risk of heart disease, heart attack and stroke. (American Cancer Society, “Smokeless Tobacco” 2010) Smokeless tobacco products contain at least 28 cancer-causing agents. The risk of certain types of cancer increases with smokeless tobacco: Esophageal cancer, oral cancer (cancer of the mouth, throat, cheek, gums, lips, tongue). Other Tobacco Products (OTP) Include: Chewing/Spit Tobacco A smokeless tobacco product consumed by placing a portion of the tobacco between the cheek and gum or upper lip teeth and chewing. Must be manually crushed with the teeth to release flavor and nicotine. Spitting is required to get rid of the unwanted juices. Loose Tobacco Loose (pipe) tobacco is made of cured and dried leaves; often a mix of various types of leaves (including spiced leaves), with sweeteners and flavorings added to create an "aromatic" flavor. The tobacco used resembles cigarette tobacco, but is more moist and cut more coarsely. Pipe smoke is usually held in the mouth and then exhaled without inhaling into the lungs. Blunt Wraps Blunt wraps are hollowed out tobacco leaf to be filled by the consumer with tobacco (or other drugs) and comes in different flavors. Flavors are added to create aromas and flavors. -

Smoking Behavior and the Use of Cigarette Types Among University Student
E-ISSN 2240-0524 Journal of Educational and Social Research Vol 10 No 5 ISSN 2239-978X www.richtmann.org September 2020 . Research Article © 2020 Arisona et.al.. This is an open access article licensed under the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/) Smoking Behavior and the Use of Cigarette Types Among University Student Amalia Arisona Laili Rahayuwati Ayu Prawesti Habsyah Saparidah Agustina Faculty of Nursing, Universitas Padjadjaran, Indonesia DOI: https://doi.org/10.36941/jesr-2020-0100 Abstract The number of smokers is increasing every year in Indonesia. Cigarettes can cause several health problems and can even cause death. Aside from conventional cigarettes, e-cigarettes and shisha are starting to get the spotlight. The types of cigarettes include conventional cigarettes, electric cigarettes and shisha can cause health problems to both smokers and the people around them. The purpose of this study was to determine smoking behavior and the use of cigarette types in students. This research was quantitative descriptive. The population in this study were students who have smoked either the conventional, electric or shisha. The sampling technique used was accidental sampling with 384 students as the samples. The instrument in this study used a questionnaire that was independently developed by researchers with a total of 14 questions. The results of the data obtained were then analyzed using descriptive analysis presented in the form of a percentage. Based on the results of the study it was found that non-health students (90.6%) were more likely to smoke than health students (9.4%). -

Current Status of the Reduced Propensity Ignition Cigarette Program in Hawaii
Hawaii State Fire Council Current Status of the Reduced Propensity Ignition Cigarette Program in Hawaii Submitted to The Twenty-Eighth State Legislature Regular Session June 2015 2014 Reduced Ignition Propensity Cigarette Report to the Hawaii State Legislature Table of Contents Executive Summary .…………………………………………………………………….... 4 Purpose ..………………………………………………………………………....................4 Mission of the State Fire Council………………………………………………………......4 Smoking-Material Fire Facts……………………………………………………….............5 Reduced Ignition Propensity Cigarettes (RIPC) Defined……………………………......6 RIPC Regulatory History…………………………………………………………………….7 RIPC Review for Hawaii…………………………………………………………………….9 RIPC Accomplishments in Hawaii (January 1 to June 30, 2014)……………………..10 RIPC Future Considerations……………………………………………………………....14 Conclusion………………………………………………………………………….............15 Bibliography…………………………………………………………………………………17 Appendices Appendix A: All Cigarette Fires (State of Hawaii) with Property and Contents Loss Related to Cigarettes 2003 to 2013………………………………………………………18 Appendix B: Building Fires Caused by Cigarettes (State of Hawaii) with Property and Contents Loss 2003 to 2013………………………………………………………………19 Appendix C: Cigarette Related Building Fires 2003 to 2013…………………………..20 Appendix D: Injuries/Fatalities Due To Cigarette Fire 2003 to 2013 ………………....21 Appendix E: HRS 132C……………………………………………………………...........22 Appendix F: Estimated RIPC Budget 2014-2016………………………………...........32 Appendix G: List of RIPC Brands Being Sold in Hawaii………………………………..33 2 2014 -

Heated Cigarettes: How States Can Avoid Getting Burned
HEATED CIGARETTES: HOW STATES CAN AVOID GETTING BURNED 8/30/18 1 HEATED CIGARETTES HOW STATES CAN AVOID GETTING BURNED 8/30/18 2 THE PUBLIC HEALTH LAW CENTER 8/30/18 3 8/30/18 4 LEGAL TECHNICAL ASSISTANCE Legal Research Policy Development, Implementation, Defense Publications Trainings Direct Representation Lobby 8/30/18 5 HEATED CIGARETTES HOW STATES CAN AVOID GETTING BURNED • Presenters: – Kristy Marynak, MPP, Public Health Analyst, Centers for Disease Control and Prevention – Hudson Kingston, JD, LLM, Staff Attorney, Tobacco Control Legal Consortium at the Public Health Law Center 8/30/18 6 HEATED CIGARETTES HOW STATES CAN AVOID GETTING BURNED • Heated cigarettes on the global market • Distinguishing features 1. Heating at a temperature lower than conventional cigarettes that produce an inhalable aerosol • Heated Cigarettes: 450-700° F (generally) • Conventional cigarettes: 1250 – 1300 °F, • (max: 1500 °F) 2. Processed, commercial tobacco leaf is the nicotine source, flavor source, or both 8/30/18 7 HEATED CIGARETTES HOW STATES CAN AVOID GETTING BURNED Federal Regulation • Pre-Market Review • Modified Risk Tobacco Product Application • Vapeleaf 8/30/18 8 Heated Tobacco Products: Considerations for Public Health Policy and Practice KRISTY MARYNAK, MPP LEAD PUBLIC HEALTH ANALYST CDC OFFICE ON SMOKING AND HEALTH TOBACCO CONTROL LEGAL CONSORTIUM WEBINAR AUGUST 2018 8/30/18 9 What’s the public health importance of this topic? The landscape of tobacco products is continually changing By being proactive and anticipating new products, we can -

Economic Analysis of the EU Market of Tobacco, Nicotine and Related Products
Executive Agency for Health and Consumers Specific Request EAHC/2011/Health/11 for under EAHC/2010/Health/01 Lot 2 Economic analysis of the EU market of tobacco, nicotine and related products Revised Final Report 20 September 2013 Economic analysis of the EU market of tobacco, nicotine and related products Disclaimer This report was produced under the Health Programme (2008-13) in the frame of a contract with the Executive Agency for Health and Consumers (EAHC) acting on behalf of the European Commission. The content of this report represents the views of Matrix Insight and is its sole responsibility; it can in no way be taken to reflect the views of the European Commission and/or EAHC or any other body of the European Union. The European Commission and/or EAHC do not guarantee the accuracy of the data included in this report, nor do they accept responsibility for any use made by third parties thereof. In keeping with our values of integrity and excellence, Matrix has taken reasonable professional care in the preparation of this report. Although Matrix has made reasonable efforts to obtain information from a broad spectrum of sources, we cannot guarantee absolute accuracy or completeness of information/data submitted, nor do we accept responsibility for recommendations that may have been omitted due to particular or exceptional conditions and circumstances. © Matrix Insight Ltd, 2009 Any enquiries about this report should be directed to [email protected] Matrix Insight Ltd. | 20 September 2013 2 Economic analysis of the EU -

"I Always Thought They Were All Pure Tobacco'': American
“I always thought they were all pure tobacco”: American smokers’ perceptions of “natural” cigarettes and tobacco industry advertising strategies Patricia A. McDaniel* Department of Social and Behavioural Sciences, School of Nursing University of California, San Francisco 3333 California Street, Suite 455 San Francisco, CA 94118 USA work: (415) 514-9342 fax: (415) 476-6552 [email protected] Ruth E. Malone Department of Social and Behavioral Sciences, School of Nursing University of California, San Francisco, USA *Corresponding author The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Tobacco Control editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://tc.bmj.com/misc/ifora/licence.pdf). keywords: natural cigarettes, additive-free cigarettes, tobacco industry market research, cigarette descriptors Word count: 223 abstract; 6009 text 1 table, 3 figures 1 ABSTRACT Objective: To examine how the U.S. tobacco industry markets cigarettes as “natural” and American smokers’ views of the “naturalness” (or unnaturalness) of cigarettes. Methods: We reviewed internal tobacco industry documents, the Pollay 20th Century Tobacco Ad Collection, and newspaper sources, categorized themes and strategies, and summarized findings. Results: Cigarette advertisements have used the term “natural” since at least 1910, but it was not until the 1950s that “natural” referred to a core element of brand identity, used to describe specific product attributes (filter, menthol, tobacco leaf). -

Pyramid Cigarettes
** Pyramid Cigarettes ** Pyramid Red Box 10 Carton Pyramid Blue Box 10 Carton Pyramid Menthol Gold Box 10 Carton Pyramid Menthol Silver Box 10 Carton Pyramid Orange Box 10 Carton Pyramid Red Box 100 10 Carton Pyramid Blue Box 100 10 Carton Pyramid Menthol Gold Box 100 10 Carton Pyramid Menthol Silver Box 100 10 Carton Pyramid Orange Box 100 10 Carton Pyramid Non Filter Box 10 Carton ** E Cigarettes ** Logic Disposable E Cigarette Menthol Gold 24 Box Logic Disposable E Cigarette Menthol High 24 Box Logic Disposable E Cigarette Menthol Platinum 24 Box Logic Disposable E Cigarette Menthol Sterling 24 Box Logic Disposable E Cigarette Menthol Zero 24 Box Logic Disposable E Cigarette Gold 24 Box Logic Disposable E Cigarette High 24 Box Logic Disposable E Cigarette Sterling 24 Box Logic Disposable E Cigarette Platinum 24 Box Logic Disposable E Cigarette Zero 24 Box ** Premium Cigars ** Acid Krush Classic Blue 5-10pk Tin Acid Krush Classic Mad Morado 5-10pk Tin Acid Krush Classic Gold 5-10pk Tin Acid Krush Classic Red 5-10pk Tin Acid Kuba Kuba 24 Box Acid Blondie 40 Box Acid C-Note 20 Box Acid Kuba Maduro 24 Box Acid 1400cc 18 Box Acid Blondie Belicoso 24 Box Acid Kuba Deluxe 10 Box Acid Cold Infusion 24 Box Ambrosia Clove Tiki 10 Box Acid Larry 10-3pk Pack Acid Deep Dish 24 Box Acid Wafe 28 Box Acid Atom Maduro 24 Box Acid Nasty 24 Box Acid Roam 10 Box Antano Dark Corojo Azarosa 20 Box Antano Dark Corojo El Martillo 20 Box Antano Dark Corojo Pesadilla 20 Box Antano Dark Corojo Poderoso 20 Box Natural Dirt 24 Box Acid Liquid 24 Box Acid Blondie -

Perception Des Fumeurs Et Non Fumeurs Des Cigarettes Actuelles Vs Cigarettes Neutres
Perception des fumeurs et non fumeurs des cigarettes actuelles vs cigarettes neutres Emmanuelle Béguinot et Figen Eker, Comité National Contre le Tabagisme (CNCT), Paris Yves Martinet, CHU et Université Henri Poincaré, Nancy Karine Gallopel-Morvan, Maître de Conférences, Ecole des Hautes Etudes en Santé Publique (EHESP), Rennes ----- Journée Internationale du Marketing Santé 23/03/2012 Le tabac en France : contexte 28,7 % fument quotidiennement (INPES, 2010) 60 000 décès chaque année Interdiction de la publicité - Loi Evin (1991) • p.2 Le paquet de cigarettes est devenu le support publicitaire • p.3 • p.4 Packagings neutres (art.11, CCLAT) • p.5 Impact des paquets neutres des produits du tabac ? Beede, Lawson, Shepherd (1991), The promotional impact of cigarette packaging: a study of adolescent responses to cigarette plain-packs, paper presented to the Australian and New Zealand Association and management Educator’s conference, Launceston, Australia. Beede and Lawson (1992), The effect of plain packaging on the perception of health warnings, Public health, 106 (4), 315-322. Beede and Lawson (1991), Brand image attraction: the promotional impact of cigarette packaging, The New Zealand family physician, 18, 175-177 Canadian Medical Association (1994), Plain packaging of tobacco product, may 3, 1994, Ottawa (Ontario), presentation to the House of Commons Standing on Health. Canadian Cancer Society (1994), Highlights : study of the effect of plain pack packaging among youths, 6/01/1994, centre for health promotion de l’université de Toronto. Canadian Cancer Society (1994), Putting health first : the case for plain packaging of tobacco products, april, submitted to the House of Commons Standing on Health.