(Atipamezole Hydrochloride) Include Hypersalivation, Diarrhea, and Tremors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

Download Drug Labels List
Syringe Labelling System Price Per Label Description/Drug Name Item No. Quanitiy Per Pack Pack Abciximab 99801 2 x 500 roll's £6.30 Abidec 100602 2 x 500 roll's £6.30 Acepromazine 99802 2 x 500 roll's £6.30 Acetazolamide 99803 2 x 500 roll's £6.30 Acetylcholine 99804 2 x 500 roll's £6.30 Acetylcysteine 99805 2 x 500 roll's £6.30 Acetylsalicylic Acid 99806 2 x 500 roll's £6.30 Aciclovir 99807 2 x 500 roll's £6.30 ACP/Buprenorphine 100208 2 x 500 roll's £6.30 Actrapid Insulin 99808 2 x 500 roll's £6.30 Adenosine 99809 2 x 500 roll's £6.30 Adrenaline (Top Half Black, Bottom Violet, Violet Text) 99810 2 x 500 roll's £6.30 Adrenaline/Epinephrine 99811 2 x 500 roll's £6.30 Albumin Solution 99812 2 x 500 roll's £6.30 Alchol 99813 2 x 500 roll's £6.30 Alemtuzmab 99814 2 x 500 roll's £6.30 ALERT 100243 2 x 500 roll's £6.30 Alfaxalone 99815 2 x 500 roll's £6.30 Alfentanil 99816 2 x 500 roll's £6.30 Alfentanil 99817 2 x 500 roll's £6.30 Alteplase 99818 2 x 500 roll's £6.30 Amikacin 99819 2 x 500 roll's £6.30 Aminophylline 99820 2 x 500 roll's £6.30 Amiodarone 100194 2 x 500 roll's £6.30 Amoxicillin 100195 2 x 500 roll's £6.30 Amphotericin 99821 2 x 500 roll's £6.30 Ampicillin 99822 2 x 500 roll's £6.30 Antibiotic 99823 2 x 500 roll's £6.30 Anticoagulant 99824 2 x 500 roll's £6.30 Antifungal 100228 2 x 500 roll's £6.30 Antiseptic 99825 2 x 500 roll's £6.30 Aprotinin 99826 2 x 500 roll's £6.30 Aqueous Iodine 99827 2 x 500 roll's £6.30 Arterial 100259 2 x 500 roll's £6.30 Arterial ( Line Label - White with Red Writing) 100176 2 x 500 roll's £6.30 Arterial -

Low Doses of A2-Adrenoceptor Antagonists Augment Spinal Morphine Analgesia and Inhibit Development of Acute and Chronic Tolerance
British Journal of Pharmacology (2008) 155, 1264–1278 & 2008 Macmillan Publishers Limited All rights reserved 0007– 1188/08 $32.00 www.brjpharmacol.org RESEARCH PAPER Low doses of a2-adrenoceptor antagonists augment spinal morphine analgesia and inhibit development of acute and chronic tolerance B Milne, M Sutak, CM Cahill and K Jhamandas Department of Pharmacology and Toxicology, and Department of Anesthesiology, Queen’s University, Kingston, Ontario, Canada Background and purpose: Ultra-low doses of opioid receptor antagonists augment spinal morphine antinociception and block the induction of tolerance. Considering the evidence demonstrating functional and physical interactions between the opioid and a2-adrenoceptors, this study investigated whether ultra-low doses of a2-adrenoceptor antagonists also influence spinal morphine analgesia and tolerance. Experimental approach: Effects of low doses of the competitive a2-adrenoceptor antagonists—atipamezole (0.08, 0.8 ng), yohimbine (0.02, 2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) were investigated on intrathecal morphine analgesia, as well as acute and chronic morphine antinociceptive tolerance using the rat tail flick and paw pressure tests. Key results: At doses markedly lower than those producing a2-adrenoceptor blockade, atipamezole, yohimbine, mirtazapine and idazoxan, prolonged the antinociceptive effects of morphine. When co-administered with repeated acute spinal injections of morphine, all four agents blocked the induction of acute tolerance. Co-injection of atipamezole with morphine for 5 days inhibited the development of tolerance in a chronic treatment paradigm. Spinal administration of atipamezole also reversed established antinociceptive tolerance to morphine as indicated by the restoration of morphine antinociceptive potency. The effects of atipamezole on spinal morphine tolerance were not influenced by treatment with 6-hydroxydopamine. -

Supplementary Material Final Submitted
Supplementary material S1 Ciprofloxacin, Danofloxacin, Difloxacin, Enrofloxacin, Flumequine, Marbofloxacin, Nalidixic acid, Norfloxacin, Oxolinic acid, Sarafloxacin, Sulfaguanidine, Sulfanilamide, Sulfachlorpyridazine, Sulfadiazine, Sulfadimethoxine, Sulfadoxine, Sulfamerazine, Sulfamethazine, Sulfamethizole, Sulfamethoxypyridazine, Sulfamonomethoxine, Sulfapyridine, Sulfaquinoxaline, Sulfathiazole, Sulfisoxazole, Sulfamoxole, Trimethoprim, Sulfamethoxazole, Erythromycin, Josamycin, Lincomycin, Spiramycin, Tilmicosin, Tylosin A, Gamithromycin, Tildipirosin, Tulathromycin, Virginiamycin, Pirlimycin, Acetyltylosin-2-O, Tylvalosin, Valnemulin, Rifampicin, Dapsone, Chlortetracycline, Epichlortetracycline, Epitetracycline, Oxytetracycline, Epioxytetracycline, Doxycycline,Tetracycline, Phenoxymethylpenicillin, Benzylpenicillin, Amoxicillin, Ampicillin, Dicloxacillin, Cloxacillin, Nafcillin, Oxacillin, Cefalexin, Cefalonium, Cefapirin, Cefoperazone, Desacetylcefapirin, Cefazolin, Cefquinome, Ceftiofur. S2 1,2-dichlorobenzene, 16ß-hydroxystanozolol, 17a-nortestosterone, 17a-trenbolone, 17ß-estradiol, 17ß-trenbolone, 2 4 6-triaminopyrimidine-5-carbonitrile, 2 4-dimethylaniline, 2ß-hydroxy-N- deethyltiamulin, 2ß-hydroxytiamulin, 3-O-acetyltylosin, 4 4'-dinitrocarbanilide/Nicarbazin (500) , 4- demethylgriseofulvin, 4-methylaminoantipyrine, 5-hydroxyflunixin, 5-hydroxythiabendazole, 6- demethylgriseofulvin, 8a-hydroxymutilin, 8a-hydroxy-N-deethyltiamulin, 8a-hydroxytiamulin, Abamectin B1a, Acepromazine, Acetylisovaleryltylosin, Acyclovir, AHD, Aklomide, -

Non-Clinical Review(S) Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209830Orig1s000 NON-CLINICAL REVIEW(S) DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR DRUG EVALUATION AND RESEARCH PHARMACOLOGY/TOXICOLOGY NDA REVIEW AND EVALUATION Application number: 209830 Supporting document/s: 1, 4, 9, 19 Applicant’s letter date: 8/31/17, 11/6/17, 11/22/17, 3/12/18 CDER stamp date: 8/31/17, 11/6/17, 11/22/17, 3/12/18 Product: ARISTADA INITIO Aripiprazole lauroxil NanoCrystal® Dispersion (AL-NCD, aripiprazole lauroxil nano and ALKS 9072N) Indication: As a starting dose to initiate ARISTADA® (aripiprazole lauroxil) treatment for schizophrenia (b) (4) Applicant: Alkermes, Inc. Review Division: Psychiatry Products Reviewer: Amy M. Avila, PhD Supervisor/Team Leader: Aisar Atrakchi, PhD Division Director: Mitchell Mathis, MD Project Manager: Kofi Ansah, PharmD Template Version: September 1, 2010 Disclaimer Except as specifically identified, all data and information discussed below and necessary for approval of NDA 209830 are owned by Alkermes or are data for which Alkermes has obtained a written right of reference. Any information or data necessary for approval of NDA 209830 that Alkermes does not own or have a written right to reference constitutes one of the following: (1) published literature, or (2) a prior FDA finding of safety or effectiveness for a listed drug, as reflected in the drug’s approved labeling. Any data or information described or referenced below from reviews or publicly available summaries of a previously approved application is for descriptive purposes only and is not relied upon for approval of NDA 209830. -

Detection of A2-Adrenergic Receptors in Brain of Living Pig with 11C-Yohimbine
Detection of a2-Adrenergic Receptors in Brain of Living Pig with 11C-Yohimbine Steen Jakobsen1, Kasper Pedersen1, Donald F. Smith2, Svend B. Jensen1, Ole L. Munk1, and Paul Cumming1 1Aarhus University PET Centre and Centre for Functionally Integrative Neuroscience, Aarhus, Denmark; and 2Center for Basic Psychiatric Research, Psychiatric Hospital of Aarhus University, Risskov, Denmark been developed for PET studies for receptors and reuptake There have been few radiotracers for imaging adrenergic recep- sites of the biogenic amines dopamine, serotonin, and tors in brain by PET, but none has advanced for use in human noradrenaline (2,3). Altered noradrenergic transmission is studies. We developed a radiosynthesis for the a2-adrenergic implicated in several neurodegenerative diseases and in the antagonist 11C-yohimbine and characterized its binding in living mechanism of action of some antidepressants. However, pigs. As a prelude to human studies with 11C-yohimbine, we determined the whole-body distribution of 11C-yohimbine and radiotracers for markers of noradrenaline innervations calculated its dosimetry. Methods: Yorkshire · Landrace pigs and receptors have become available only in recent years. weighing 35–40 kg were used in the study. Baseline and post- Plasma membrane noradrenaline transporters on presynap- challenge PET recordings of 11C-yohimbine in pig brain were tic terminals can now be imaged with 11C-labeled 2-[(2- conducted for 90 min, concurrent with arterial blood sampling, methoxyphenoxy)phenylethyl]morpholine (11C-MeNER) (4). and with yohimbine and RX821002 as pharmacologic interven- Whereas specific radiotracers are not available for imaging of tions. 15O-Water scans were performed to detect changes in brain b-adrenergic receptors, a -adrenergic receptors in brain cerebral perfusion. -
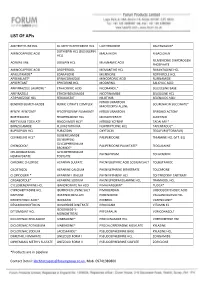
LIST of Apis
LIST OF APIs AMITRIPTYLINE HCL DL-METHYLEPHEDRINE HCL LIOTHYRONINE RALTEGRAVIR* DOTHIEPIN HCL (DOSULEPIN AMINOCAPROIC ACID MALATHION R-BACLOFEN HCL) RILMENIDINE DIHYDROGEN ACRIVASTINE DOXEPIN HCL MEFENAMIC ACID PHOSPHATE AMINOCAPROIC ACID DROPERIDOL MEMANTINE HCL RIMANTADINE HCL APALUTAMIDE* EDARAVONE MILRINONE ROPINIROLE HCL APREMILAST* EFINACONAZOLE MINODRONIC ACID RUFINAMIDE APREPITANT EPHEDRINE HCL MODAFINIL SALICYLIC ACID ARIPIPRAZOLE LAUROXIL* ETHACRYNIC ACID NICORANDIL* SELEGELINE BASE ARIPIRAZOLE ETHOXYBENZAMIDE NICOTINAMIDE SELEGILINE HCL ATIPAMEZOLE HCL FEBUXOSTAT NILOTINIB SELENIUOS ACID NITROFURANTOIN BENDROFLUMETHIAZIDE FERRIC CITRATE COMPLEX SOLIFENACIN SUCCINATE* MACROCRYSTALLINE BENZYL BENZOATE FESOTERODINE FUMARATE NITROFURANTOIN SPIRONOLACTONE BORTEZOMIB FEXOFENADINE HCL MONOHYDRATE SUNITINIB BRETYLIUM TOSYLATE FINGOLIMOD HCL* NITROGLYCERINE TADALAFIL* BRINZOLAMIDE FLUVASTATIN NA NORTRIPTLYINE HCL TAPENTADOL* BUPROPION HCL FURAZIDIN OXYTOCIN TEGAFUR (FTORAFUR) GLIBENCLAMIDE CEVIMELINE HCL* PALIPERIDONE THIAMINE HCL (VIT. B1) (GLYBURIDE) GLYCOPYRRONIUM CHENODIOL* PALIPERIDONE PALMITATE* TIOGUANINE BROMIDE* CHLOROBUTANOL GLYCOPYRRONIUM PHENAZEPAM TOLAZAMIDE HEMIHYDRATE TOSYLATE CHROMIC CHLORIDE HEPARAN SULFATE PHENYLBUTYRIC ACID SODIUM SALT TOLBUTAMIDE CILOSTAZOL HEPARINE CALCIUM PHENYLEPHRINE BITARTRATE TOLCAPONE CLOPIDOGREL* HEPARINE LITHIUM PHENYLEPHRINE HCL TOLTERODINE TARTRATE CRISABOROLE* HEPARINE SODIUM PHENYLPROPANOLAMINE HCL TRAMADOL HCL CYCLOBENZAPRINE HCL IBANDRONATE NA H2O PIMAVANSERIN* TUDCA* CYPROHEPTADINE -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Effect of Different Doses of Atipamezole on Reversal Of
veterinary sciences Article Effect of Different Doses of Atipamezole on Reversal of Medetomidine-Induced Tear-Flow Decrease in Rats Teppei Kanda 1,2,* , Manami Gotoh 2, Ayumi Makino 2, Kayo Furumoto 1,2, Yuki Shimizu 1,2, Takamasa Itoi 1,2, Noritaka Maeta 1,2 and Toshinori Furukawa 2,3 1 Faculty of Veterinary Medicine, Okayama University of Science, 1-3 Ikoino-oka, Imabari, Ehime 794-8555, Japan; [email protected] (K.F.); [email protected] (Y.S.); [email protected] (T.I.); [email protected] (N.M.) 2 Department of Comparative Animal Science, College of Life Science, Kurashiki University of Science and the Arts, 2640 Nishinoura, Tsurajima-cho, Kurashiki, Okayama 712-8505, Japan; [email protected] (M.G.); [email protected] (A.M.); [email protected] (T.F.) 3 Department of Animal Pharmaceutical Science, School of Pharmaceutical Science, Kyushu University of Health and Welfare, 1714-1 Yoshino-cho, Nobeoka, Miyazaki 882-8508, Japan * Correspondence: [email protected] Received: 3 November 2020; Accepted: 2 December 2020; Published: 3 December 2020 Abstract: It has been reported that α2-adrenoceptor agonists such as medetomidine decrease tear flow in many species, including rats. Few studies have investigated the involvement of α2-adrenoceptor in decreased tear flow; the issue has not been illustrated sufficiently. Therefore, we aimed to investigate the effect of different doses of atipamezole on the reversal of medetomidine-induced tear-flow decrease to reveal the specific involvement of α2-adrenoceptor. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Small Animal
Small Animal Sampler Separation Distress Syndrome From Blackwell’s Five-Minute Veterinary Consult – Canine and Feline, Sixth Edition. by Deborah F. Horwitz Chapter 236: Vomiting From The Feline Patient, Fifth Edition. Edited by Gary D. Norsworthy. Chapter 12: Pharmacologic and Clinical Principles of Adjunct Analgesia From Analgesia and Anesthesia for the Ill or Injured Dog and Cat, First Edition. by Karol A. Mathews, Melissa Sinclair, Andrea M. Steele, and Tamara Grubb. 1208 Blackwell’s Five-Minute Veterinary Consult Separation Distress Syndrome commonly reported. Destruction targets windows and doors and/or owner possessions. Other signs include behavioral depression, BASICS anorexia,r drooling, hiding, shaking, panting, DIAGNOSIS DEFINITION pacing, attempts to prevent owner departure, DIFFERENTIAL DIAGNOSIS A distress response of dogs (occasionally cats) and self-trauma from lick lesions. Diarrhea Vocalization: response to outdoor separated from the person or persons to and vomiting are occasionally noted. Signs influences,r territorial displays, play with other whom they are most attached, usually their of strong pet-owner attachment may ber pets in the home or fears. Destructive owner(s). The separation may be real (the present: excessive attention-seeking behaviors behaviors: occur both whenr the owner is owner is gone) or perceived (the pet is just and following behaviors but not necessary for present and absent (e.g., territorial destructive separated from the owner). In other cases the diagnosis. Frequently owners report displays at windows and doors; destruction pet may be distressed because some excessive, excited,r and prolonged greeting due to fear-producing stimuli such as noises fear-inducing event has occurred while home behavior upon return. -

No Role of Beta Receptors in Cognitive Flexibility: Evidence from a Task-Switching Paradigm in a Randomized Controlled Trial
Neuroscience 295 (2015) 237–242 NO ROLE OF BETA RECEPTORS IN COGNITIVE FLEXIBILITY: EVIDENCE FROM A TASK-SWITCHING PARADIGM IN A RANDOMIZED CONTROLLED TRIAL L. STEENBERGEN, * R. SELLARO, M. DE ROVER, in an attentional set-shifting task (Lapiz and Morilak, B. HOMMEL AND L. S. COLZATO 2006). Comparable improvements in set shifting have Institute for Psychological Research, Leiden University, Leiden, been achieved by chronic treatment with desipramine, The Netherlands an NE-reuptake blocker, and atipamezole, an a-2-adre- Leiden Institute for Brain and Cognition, Leiden University, nergic autoreceptor antagonist (Lapiz et al., 2007; Bondi Leiden, The Netherlands et al., 2010). Moreover, lesions of the dorsal noradrenergic bundle created by 6-hydroxydopamine, which causes sub- stantial depletions of cortical NE, seem to selectively Abstract—There is evidence that noradrenergic coeruleo- impair set shifting (Tait et al., 2007). Last, specific decre- cortical projections are involved in different forms of cogni- ments in attentional set-shifting task were obtained in rats tive flexibility. So far, no studies in humans have investigated after noradrenergic but not cholinergic deafferentiation the involvement of beta receptors on task-switching perfor- mance, a well-established measure of cognitive flexibility. (McGaughy et al., 2008). In humans, NE reuptake inhibi- The present study investigated whether the administration tors, such as atomoxetine, have been suggested as poten- of propranolol (a central and peripheral beta-adrenergic tial cognitive enhancers in schizophrenia because of their antagonist) affected switching costs (i.e., the increase of capacity to indirectly but selectively increase extracellular reaction time in task-switching trials relative to task- dopamine concentrations in the prefrontal cortex repetition trials).