Anisoptera: Aeshnidae)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Spatiotemporal Pattern of Phenology Across Geographic Gradients in Insects
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2017 Spatiotemporal pattern of phenology across geographic gradients in insects Khelifa, Rassim Abstract: Phenology – the timing of recurrent biological events – influences nearly all aspects of ecology and evolution. Phenological shifts have been recorded in a wide range of animals and plants worldwide during the past few decades. Although the phenological responses differ between taxa, they may also vary geographically, especially along gradients such as latitude or elevation. Since changes in phenology have been shown to affect ecology, evolution, human health and the economy, understanding pheno- logical shifts has become a priority. Although phenological shifts have been associated with changes in temperature, there is still little comprehension of the phenology-temperature relationship, particularly the mechanisms influencing its strength and the extent to which it varies geographically. Such ques- tions would ideally be addressed by combining controlled laboratory experiments on thermal response with long-term observational datasets and historical temperature records. Here, I used odonates (drag- onflies and damselflies) and Sepsid scavenger flies to unravel how temperature affects development and phenology at different latitudes and elevations. The main purpose of this thesis is to provide essential knowledge on the factors driving the spatiotemporal phenological dynamics by (1) investigating how phenology changed in time and space across latitude and elevation in northcentral Europe during the past three decades, (2) assessing potential temporal changes in thermal sensitivity of phenology and (3) describing the geographic pattern and usefulness of thermal performance curves in predicting natural responses. -
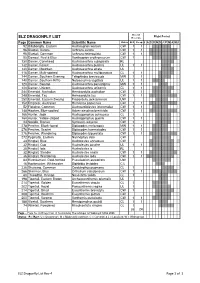
ELZ Dragonfly List Rev 4.Xlsx
Recent Flight Period ELZ DRAGONFLY LIST Records Page Common Name Scientific Name Status ELZ Co-op JASONDJFMAMJ 92 Billabongfly, Eastern Austroagrion watsoni CW 1 1 96 Bluetail, Aurora Ischnura aurora CW 1 1 96 Bluetail, Common Ischnura heterosticta CW 1 1 90 Damsel, Red & Blue Xanthagrion erythroneurum CW 1 130 Darner, Conehead Austroaeschna subapicalis RL 132 Darner, Forest Austroaeschna pulchra UL 1 1 130 Darner, Mountain Austroaeschna atrata UL 116 Darner, Multi-spotted Austroaeschna multipunctata CL 1 1 144 Darner, Southern Evening Telephlebia brevicauda MW 1 1 140 Darner, Southern Riffle Notoaeschna sagittata UL 1 1 120 Darner, Swamp Austroaeschna parvistigma MW 1 1 124 Darner, Unicorn Austroaeschna unicornis CL 1 1 244 Emerald, Australian Hemicordulia australiae CW 1 1 248 Emerald, Tau Hemicordulia tau CW 1 1 250 Emerald, Eastern Swamp Procordulia jacksoniensis UW 152 Emperor, Australian Hemianax papuensis CW 1 1 52 Flatwing, Common Austroargiolestes icteromelas CW 1 1 148 Hawker, Blue-spotted Adversaeschna brevistyla CW 1 1 166 Hunter, Jade Austrogomphus ochraceus CL 1 164 Hunter, Yellow-striped Austrogomphus guerini CW 1 1 24 Needle, Bronze Synlestes weyersii CW 1 278 Percher, Black-faced Diplacodes melanopsis MW 1 1 276 Percher, Scarlet Diplacodes haematodes CW 1 1 276 Percher, Wandering Diplacodes bipunctata CW 1 1 272 Pygmyfly, Eastern Nannophya dalei CW 34 Ringtail, Blue Austrolestes annulosus CW 32 Ringtail, Cup Austrolestes psyche UL 1 1 36 Ringtail, Iota Austrolestes io RL 32 Ringtail, Slender Austrolestes analis CW 1 1 -

HEXAPODA INSECTA Australia's Faunal Extinction Crisis Submission
SUPPORTING INFORMATION Table S3 Australian insects and allied invertebrates that have been listed under various conservation schedules, including State/Territory Acts, the EPBC Act and the IUCN Red List, and their occurrence in IBRA regions. Listed species Conservation status Conservation status Conservation status IBRA region (State) (various (EPBC Act 1999) (IUCN Red List 2017) State/Territory Acts) HEXAPODA INSECTA BLATTODEA Panesthia lata Walker, 1868, (Lord Howe Island Endangered PSI (NSW) Wood-feeding Cockroach) (Biodiversity Conservation Act 2016) COLEOPTERA Aulacopris matthewsi Storey, 1986 (Flightless Vulnerable WET (QLD) Dung Beetle) Castiarina insculpta (Carter, 1934) (Miena Jewel Endangered TCH (TAS) Beetle) (Threatened Species Protection Act 1995 Catadromus lacordairei Boisduval , 1835 (Green- Vulnerable FUR, TNM (TAS) lined Ground Beetle) (Threatened Species Protection Act 1995) Enchymus sp. nov. Pascoe, 1871 (Weldborough Rare (Threatened BEL (TAS) Forest Weevil) Species Protection Act 1995) Goedetrechus mendumae Moore, 1972 (Ida Bay Vulnerable TSR (TAS) Cave Beetle) (Threatened Species Protection Act 1995) Goedetrechus parallelus Moore, 1972 (Junee- Vulnerable TWE (TAS) Florentine Cave Beetle) (Threatened Species Protection Act 1995) Hoplogonus bornemisszai Bartolozzi, 1996 Endangered Critically Endangered BEL (TAS) (Bornemissza’s Stag Beetle) (Threatened Species Protection Act 1995 – TAS) Hoplogonus simsoni Parry, 1875 (Simsons Stag Vulnerable Vulnerable BEL, TCH (TAS) Beetle) (Threatened Species Protection Act 1995) Hoplogonus -

A Revised, ANNOTATED Checklist of Victorian Dragonflies (Insecta: Odonata)
A REVISED, ANNOTATED CHECKLIST OF Victorian DRAGONFLIES (Insecta: Odonata) I.D. ENDERS by 56 Looker Road, Montmorency, Victoria 3094, Australia E-mail: [email protected] ENDERS by , I.D. 2010. A revised, annotated checklist of the Victorian dragonflies (Insecta: Odonata). Proceedings of the Royal Society of Victoria. 122(1): 9-27. ISSN 0035-9211. Seventy-six species of Odonata are known from Victoria (26 Zygoptera; 50 Anisoptera). In the last ten years one new species Austroaeschna ingrid Theischinger, 2008 has been described from the State; Austroepigomphus praeruptus (Selys, 1857) and Pseudagrion microcephalum (Rambur, 1842) have now been recorded; and records of Rhadinosticta banksi (Tillyard, 1913) and Labidiosticta vallisi (Fraser, 1955) are judged to be erroneous. Generic names of Aeshna, and Trapezostigma have been changed. Some changes in higher level names and relationships, based on recent phylogenetic analyses, have been incorporated. Distribution maps for all species, based on museum collections, are provided. Key Words: Odonata, Zygoptera, Anisoptera, Victoria, Australia, checklist, Hemiphlebia IN the ten years since an annotated checklist of the molecular study seeks greater taxon and genome Victorian Odonata was published (Endersby 2000), sampling and, as this occurs, slow convergence be- a new species has been described from Victoria, tween the alternatives is appearing. In the meantime additional species have been recorded in the State, some framework is needed on which to list the Vic- substantial museum collection label data have be- torian fauna today. Theischinger & Endersby (2009) come available, and numerous phylogenetic stud- have tried to steer a middle course, avoiding the ex- ies have been published. Theischinger & Endersby tremes but acknowledging that change is occurring; (2009) have incorporated many of these changes into it will still annoy some but must be seen as a work in an identification guide for the adults and larvae of progress. -

Dragonflies of the Soutpansberg
DRAGONFLIES 43 DRAGONFLIES W. Tarboton Sourcesofinformation Family Lestidae Spreadwings Lestes plagiatus Highland Spreadwing To my knowledge there has been no comprehensive or systematic assessment of the dragonfly fauna of the Sout- Lestes virgatus Smoky Spreadwing pansberg. Van Son, Pinhey and others have done some Family Protoneuridae Pinflies collecting here, mostly in the 1940s and 1950s. From this, Elattoneura glauca Common Threadtail a total of 52 species from the Soutpansberg are repre- sented in South African museum collections and these are Family Platycnemididae Stream-Damsels listed below. Allocnemis leucosticta Goldtail Summarystatistics Family Coenagrionidae Sprites Ceriagrion glabrum CommonOrange This list of 52 species, comprising about a third of the known South African dragonfly fauna (which totals 159 Pseudagrion commoniae nigerrimum BlackSprite species), would undoubtedly be increased — perhaps by Pseudagrion hageni Hagen’sSprite another 30–40 species — if a dedicated dragonfly survey Pseudagrion hamoni Hamon’s Sprite of the area were to be undertaken. Given the area’s close Pseudagrion kersteni Kersten’s Sprite proximity to Zimbabwe, it is likely that one or more spe- Pseudagrion makabusiense Makabusi Sprite cies new for the South African list will be found here (e.g. Actoneura biordinata), and it is not inconceivable, given Pseudagrion massaicum MasaiSprite the mountain range’s relative isolation, that species new Pseudagrion salisburyense SalisburySprite to science could be discovered here as well. Pseudagrion spernatum NatalSprite Pseudagrion sublacteum Cherry-EyeSprite As it stands the list includes two species that are endemic Ischnura senegalensis Bluetail to South Africa (Aeshna subpupillata, Allocnemis leucosticta) and three that are listed in the recently pub- Africallagma glaucum Swamp Bluet lished dragonfly Red Data list (Aeshna ellioti — vulnera- Agriocnemis exilis Little Whisp ble; Chlorolestes elegans — vulnerable; Pseudagrion SuborderAnisoptera(Dragonflies) makabusiense — critical). -

An Overview of Molecular Odonate Studies, and Our Evolutionary Understanding of Dragonfly and Damselfly (Insecta: Odonata) Behavior
International Journal of Odonatology Vol. 14, No. 2, June 2011, 137–147 Dragons fly, biologists classify: an overview of molecular odonate studies, and our evolutionary understanding of dragonfly and damselfly (Insecta: Odonata) behavior Elizabeth F. Ballare* and Jessica L. Ware Department of Biological Sciences, Rutgers, The State University of New Jersey, 195 University Ave., Boyden Hall, Newark, NJ, 07102, USA (Received 18 November 2010; final version received 3 April 2011) Among insects, perhaps the most appreciated are those that are esthetically pleasing: few capture the interest of the public as much as vibrantly colored dragonflies and damselflies (Insecta: Odonata). These remarkable insects are also extensively studied. Here, we review the history of odonate systematics, with an emphasis on discrepancies among studies. Over the past century, relationships among Odonata have been reinterpreted many times, using a variety of data from wing vein morphology to DNA. Despite years of study, there has been little consensus about odonate taxonomy. In this review, we compare odonate molecular phylogenetic studies with respect to gene and model selection, optimality criterion, and dataset completeness. These differences are discussed in relation to the evolution of dragonfly behavior. Keywords: Odonata; mitochondrion; nuclear; phylogeny; systematic; dragonfly; damselfly Introduction Why study Odonata? The order Odonata comprises three suborders: Anisozygoptera, Anisoptera, and Zygoptera. There are approximately 6000 species of Odonata described worldwide (Ardila-Garcia & Gregory, 2009). Of the three suborders Anisoptera and Zygoptera are by far the most commonly observed and collected, because there are only two known species of Anisozygoptera under the genus Epiophlebia. All odonate nymphs are aquatic, with a few rare exceptions such as the semi-aquatic Pseudocordulia (Watson, 1983), and adults are usually found near freshwater ponds, marshes, rivers (von Ellenrieder, 2010), streams, and lakes (although some species occur in areas of mild salinity; Corbet, 1999). -

Draft Index of Keys
Draft Index of Keys This document will be an update of the taxonomic references contained within Hawking 20001 which can still be purchased from MDFRC on (02) 6024 9650 or [email protected]. We have made the descision to make this draft version publicly available so that other taxonomy end-users may have access to the information during the refining process and also to encourage comment on the usability of the keys referred to or provide information on other keys that have not been reffered to. Please email all comments to [email protected]. 1Hawking, J.H. (2000) A preliminary guide to keys and zoological information to identify invertebrates form Australian freshwaters. Identification Guide No. 2 (2nd Edition), Cooperative Research Centre for Freshwater Ecology: Albury Index of Keys Contents Contents ................................................................................................................................................. 2 Introduction ............................................................................................................................................. 8 Major Group ............................................................................................................................................ 8 Minor Group ................................................................................................................................................... 8 Order ............................................................................................................................................................. -

Accepted Version (PDF 1MB)
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Hasan, Jafar, Roy, Anindo, Chatterjee, Kaushik, & Yarlagadda, Prasad (2019) Mimicking insect wings: The roadmap to bioinspiration. ACS Biomaterials Science and Engineering, 5(7), pp. 3139-3160. This file was downloaded from: https://eprints.qut.edu.au/131248/ c Consult author(s) regarding copyright matters This work is covered by copyright. Unless the document is being made available under a Creative Commons Licence, you must assume that re-use is limited to personal use and that permission from the copyright owner must be obtained for all other uses. If the docu- ment is available under a Creative Commons License (or other specified license) then refer to the Licence for details of permitted re-use. It is a condition of access that users recog- nise and abide by the legal requirements associated with these rights. If you believe that this work infringes copyright please provide details by email to [email protected] Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1021/acsbiomaterials.9b00217 Page 1 of 57 ACS Biomaterials Science & Engineering 1 2 3 4 5 Mimicking Insect Wings: The Roadmap to 6 7 8 9 10 Bio-inspiration 11 12 13 14 15 Jafar Hasan1, Anindo Roy2, Kaushik Chatterjee2, Prasad KDV Yarlagadda1 16 17 18 1Science and Engineering Faculty, Queensland University of Technology, 2 George Street, 19 20 Brisbane, QLD 4001, Australia 21 22 23 2Department of Materials Engineering, Indian Institute of Science, C.V. -

Reported Period Exceeding They Were (1933-36) Good
Adv. Odonatol. 4 : 53-56 December 1989 On the status of rare Indian odonate species A.R. Lahiri Zoological Survey of India, New Alipur, Calcutta - 700 053, India A list of odonate species that have not been reported from India since their description before 1948 is presented. Most of the 78 species or Hills, subspecies listed were described from North Bengal or Sikkim, Khasi and Western Ghats and Nilgiris. Conservation measures on the type locali- ties of the rare dragonflies in these areas are urged. INTRODUCTION The odonate fauna of India has been explored time and again by individual the Fraser collectors, specialists on group and various survey parties. (1933-36) summarized the earlier works. Since then a numberof survey reports have followed, adding new taxa or extending the knowledge of the range of the known ones. A careful consultation of literature reveals that a good majority ofthe known species are rare and seldom reported. Very little attempt has, however, been made so far to demarcate the rare Indian odonate species and envisage effective planning for their conservation, except for the relict dragonfly Epiophlebia laidlawiTillyard, which has been declared a protected species by the Wildlife Board, Government of India. LIST OF SPECIES NOT REPORTED FROM INDIA SINCE 1948 In order to promote general awareness about the recurrence of rareness in Indian odonate species, a list of 78 species that demand special consideration from the conservation point is presented here. It refers to all taxa that have not been reported for 40 since first described in India a variableperiod exceeding years, ever they were from the but prior 1948. -

Etymology of the Dragonflies (Insecta: Odonata) Named by R.J. Tillyard, F.R.S
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by The University of Sydney: Sydney eScholarship Journals online Etymology of the Dragonfl ies (Insecta: Odonata) named by R.J. Tillyard, F.R.S. IAN D. ENDERSBY 56 Looker Road, Montmorency, Vic 3094 ([email protected]) Published on 23 April 2012 at http://escholarship.library.usyd.edu.au/journals/index.php/LIN Endersby, I.D. (2012). Etymology of the dragonfl ies (Insecta: Odonata) named by R.J. Tillyard, F.R.S. Proceedings of the Linnean Society of New South Wales 134, 1-16. R.J. Tillyard described 26 genera and 130 specifi c or subspecifi c taxa of dragonfl ies from the Australasian region. The etymology of the scientifi c name of each of these is given or deduced. Manuscript received 11 December 2011, accepted for publication 16 April 2012. KEYWORDS: Australasia, Dragonfl ies, Etymology, Odonata, Tillyard. INTRODUCTION moved to another genus while 16 (12%) have fallen into junior synonymy. Twelve (9%) of his subspecies Given a few taxonomic and distributional have been raised to full species status and two species uncertainties, the odonate fauna of Australia comprises have been relegated to subspecifi c status. Of the 325 species in 113 genera (Theischinger and Endersby eleven subspecies, or varieties or races as Tillyard 2009). The discovery and naming of these dragonfl ies sometimes called them, not accounted for above, fi ve falls roughly into three discrete time periods (Table 1). are still recognised, albeit four in different genera, During the fi rst of these, all Australian Odonata were two are no longer considered as distinct subspecies, referred to European experts, while the second era and four have disappeared from the modern literature. -

The Little Things That Run the City How Do Melbourne’S Green Spaces Support Insect Biodiversity and Promote Ecosystem Health?
The Little Things that Run the City How do Melbourne’s green spaces support insect biodiversity and promote ecosystem health? Luis Mata, Christopher D. Ives, Georgia E. Garrard, Ascelin Gordon, Anna Backstrom, Kate Cranney, Tessa R. Smith, Laura Stark, Daniel J. Bickel, Saul Cunningham, Amy K. Hahs, Dieter Hochuli, Mallik Malipatil, Melinda L Moir, Michaela Plein, Nick Porch, Linda Semeraro, Rachel Standish, Ken Walker, Peter A. Vesk, Kirsten Parris and Sarah A. Bekessy The Little Things that Run the City – How do Melbourne’s green spaces support insect biodiversity and promote ecosystem health? Report prepared for the City of Melbourne, November 2015 Coordinating authors Luis Mata Christopher D. Ives Georgia E. Garrard Ascelin Gordon Sarah Bekessy Interdisciplinary Conservation Science Research Group Centre for Urban Research School of Global, Urban and Social Studies RMIT University 124 La Trobe Street Melbourne 3000 Contributing authors Anna Backstrom, Kate Cranney, Tessa R. Smith, Laura Stark, Daniel J. Bickel, Saul Cunningham, Amy K. Hahs, Dieter Hochuli, Mallik Malipatil, Melinda L Moir, Michaela Plein, Nick Porch, Linda Semeraro, Rachel Standish, Ken Walker, Peter A. Vesk and Kirsten Parris. Cover artwork by Kate Cranney ‘Melbourne in a Minute Scavenger’ (Ink and paper on paper, 2015) This artwork is a little tribute to a minute beetle. We found the brown minute scavenger beetle (Corticaria sp.) at so many survey plots for the Little Things that Run the City project that we dubbed the species ‘Old Faithful’. I’ve recreated the map of the City of Melbourne within the beetle’s body. Can you trace the outline of Port Phillip Bay? Can you recognise the shape of your suburb? Next time you’re walking in a park or garden in the City of Melbourne, keep a keen eye out for this ubiquitous little beetle. -

Odonata: Anisoptera: Aeshnidae: Brachytroninae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 1996 Band/Volume: 0028_1 Autor(en)/Author(s): Theischinger Günther Artikel/Article: The species of Austrophlebia TILLYARD (Odonata: Anisoptra: Aeshnidae: Brachytroninae). 305-314 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Linzer biol. Beitr. 28/1 305-314 20.8.1996 The species of Austrophlebia TlLLYARD (Odonata: Anisoptera: Aeshnidae: Brachytroninae) G. THEISCHINGER Abstract: Larvae and adults of Austrophlebia TILL YARD are analysed, resulting in the description of a second species. Key words: Austrophlebia, revision, Australia. Introduction During recent studies towards a key to the last instar larvae and exuviae of the Australian dragonflies, the larvae of Austrophlebia TlLLYARD, supposedly a mono- typic genus, were found to be heterogeneous. Differences exist particularly in the shape of the prementum, generally the most use- ful character for identifying closely allied species in many aeshnid genera, most notably in Austroaeschna SELYS, the genus considered closest to Austrophlebia. Whereas marked differences were found between populations from south of latitude 21°S and populations from north of latitude 18°S, no major morphological differences were detected between single populations from inside the two regions. Further study, involving also adults of Austrophlebia, finally revealed that the two morphologically distinct groups of larvae also produce two types of adults. The dif- ferences between the two groups of populations of Austrophlebia in colouration of adults and in adult and larval morphology are of a magnitude which indicates distinctness at species level. A second species of Austrophlebia is, therefore, described below and compared with A.