Rhus Ovata S. Watson NRCS CODE: Family: Anacardiaceae (RHOV) Order: Sapindales Subclass: Rosidae Class: Magnoliopsida
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Malosma Laurina (Nutt.) Nutt. Ex Abrams
I. SPECIES Malosma laurina (Nutt.) Nutt. ex Abrams NRCS CODE: Family: Anacardiaceae MALA6 Subfamily: Anacardiodeae Order: Sapindales Subclass: Rosidae Class: Magnoliopsida Immature fruits are green to red in mid-summer. Plants tend to flower in May to June. A. Subspecific taxa none B. Synonyms Rhus laurina Nutt. (USDA PLANTS 2017) C. Common name laurel sumac (McMinn 1939, Calflora 2016) There is only one species of Malosma. Phylogenetic analyses based on molecular data and a combination of D. Taxonomic relationships molecular and structural data place Malosma as distinct but related to both Toxicodendron and Rhus (Miller et al. 2001, Yi et al. 2004, Andrés-Hernández et al. 2014). E. Related taxa in region Rhus ovata and Rhus integrifolia may be the closest relatives and laurel sumac co-occurs with both species. Very early, Malosma was separated out of the genus Rhus in part because it has smaller fruits and lacks the following traits possessed by all species of Rhus : red-glandular hairs on the fruits and axis of the inflorescence, hairs on sepal margins, and glands on the leaf blades (Barkley 1937, Andrés-Hernández et al. 2014). F. Taxonomic issues none G. Other The name Malosma refers to the strong odor of the plant (Miller & Wilken 2017). The odor of the crushed leaves has been described as apple-like, but some think the smell is more like bitter almonds (Allen & Roberts 2013). The leaves are similar to those of the laurel tree and many others in family Lauraceae, hence the specific epithet "laurina." Montgomery & Cheo (1971) found time to ignition for dried leaf blades of laurel sumac to be intermediate and similar to scrub oak, Prunus ilicifolia, and Rhamnus crocea; faster than Heteromeles arbutifolia, Arctostaphylos densiflora, and Rhus ovata; and slower than Salvia mellifera. -
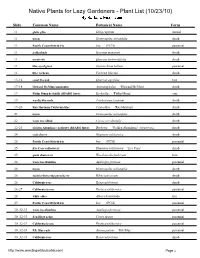
Native Plants for Lazy Gardeners - Plant List (10/23/10)
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

UNIVERSITY of CALIFORNIA Santa Barbara Ancient Plant Use and the Importance of Geophytes Among the Island Chumash of Santa Cruz
UNIVERSITY OF CALIFORNIA Santa Barbara Ancient Plant Use and the Importance of Geophytes among the Island Chumash of Santa Cruz Island, California A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Anthropology by Kristina Marie Gill Committee in charge: Professor Michael A. Glassow, Chair Professor Michael A. Jochim Professor Amber M. VanDerwarker Professor Lynn H. Gamble September 2015 The dissertation of Kristina Marie Gill is approved. __________________________________________ Michael A. Jochim __________________________________________ Amber M. VanDerwarker __________________________________________ Lynn H. Gamble __________________________________________ Michael A. Glassow, Committee Chair July 2015 Ancient Plant Use and the Importance of Geophytes among the Island Chumash of Santa Cruz Island, California Copyright © 2015 By Kristina Marie Gill iii DEDICATION This dissertation is dedicated to my Family, Mike Glassow, and the Chumash People. iv ACKNOWLEDGEMENTS I am indebted to many people who have provided guidance, encouragement, and support in my career as an archaeologist, and especially through my undergraduate and graduate studies. For those of whom I am unable to personally thank here, know that I deeply appreciate your support. First and foremost, I want to thank my chair Michael Glassow for his patience, enthusiasm, and encouragement during all aspects of this daunting project. I am also truly grateful to have had the opportunity to know, learn from, and work with my other committee members, Mike Jochim, Amber VanDerwarker, and Lynn Gamble. I cherish my various field experiences with them all on the Channel Islands and especially in southern Germany with Mike Jochim, whose worldly perspective I value deeply. I also thank Terry Jones, who provided me many undergraduate opportunities in California archaeology and encouraged me to attend a field school on San Clemente Island with Mark Raab and Andy Yatsko, an experience that left me captivated with the islands and their history. -

Native Nebraska Woody Plants
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS NATIVE NEBRASKA WOODY PLANTS Trees (Genus/Species – Common Name) 62. Atriplex canescens - four-wing saltbrush 1. Acer glabrum - Rocky Mountain maple 63. Atriplex nuttallii - moundscale 2. Acer negundo - boxelder maple 64. Ceanothus americanus - New Jersey tea 3. Acer saccharinum - silver maple 65. Ceanothus herbaceous - inland ceanothus 4. Aesculus glabra - Ohio buckeye 66. Cephalanthus occidentalis - buttonbush 5. Asimina triloba - pawpaw 67. Cercocarpus montanus - mountain mahogany 6. Betula occidentalis - water birch 68. Chrysothamnus nauseosus - rabbitbrush 7. Betula papyrifera - paper birch 69. Chrysothamnus parryi - parry rabbitbrush 8. Carya cordiformis - bitternut hickory 70. Cornus amomum - silky (pale) dogwood 9. Carya ovata - shagbark hickory 71. Cornus drummondii - roughleaf dogwood 10. Celtis occidentalis - hackberry 72. Cornus racemosa - gray dogwood 11. Cercis canadensis - eastern redbud 73. Cornus sericea - red-stem (redosier) dogwood 12. Crataegus mollis - downy hawthorn 74. Corylus americana - American hazelnut 13. Crataegus succulenta - succulent hawthorn 75. Euonymus atropurpureus - eastern wahoo 14. Fraxinus americana - white ash 76. Juniperus communis - common juniper 15. Fraxinus pennsylvanica - green ash 77. Juniperus horizontalis - creeping juniper 16. Gleditsia triacanthos - honeylocust 78. Mahonia repens - creeping mahonia 17. Gymnocladus dioicus - Kentucky coffeetree 79. Physocarpus opulifolius - ninebark 18. Juglans nigra - black walnut 80. Prunus besseyi - western sandcherry 19. Juniperus scopulorum - Rocky Mountain juniper 81. Rhamnus lanceolata - lanceleaf buckthorn 20. Juniperus virginiana - eastern redcedar 82. Rhus aromatica - fragrant sumac 21. Malus ioensis - wild crabapple 83. Rhus copallina - flameleaf (shining) sumac 22. Morus rubra - red mulberry 84. Rhus glabra - smooth sumac 23. Ostrya virginiana - hophornbeam (ironwood) 85. Rhus trilobata - skunkbush sumac 24. Pinus flexilis - limber pine 86. Ribes americanum - wild black currant 25. -

Thehortreport
THE HORT REPORT NEWSLETTER OF THE HORTICULTURAL SOCIETY OF MARYLAND, INC. | MAY 2017 CAPITAL GARDENS: Dedicated Gardeners & Creative Spaces in Annapolis ive splendid gardens of Annapolis—ranging from cottage gardens to a Zen retreat—will be on display FSunday, June 4, on the Horticultural Society of Maryland’s annual tour. The tour, from 10 a.m. to 4 p.m., will happen rain or shine. HSM members are admitted free with a current membership card. Non-member tickets cost $35 if purchased by June 3, either online (www.mdhorticulture.org) or at these locations: Kingsdene Nurseries, Monkton, The Perennial Farm, Glen Arm, Green Fields Nursery, Baltimore, Clark’s Ace Hardware, Ellicott City, and Graul’s Markets in Annapolis, Cape St. Claire, Ruxton, Mays Chapel and Hereford. On the day of the tour, non-member tickets will cost $40 and will be available at the first garden, 356 Broadview Lane, Annapolis 21401. Detailed descriptions of the gardens are available in the tour booklet that accompanies this newsletter. photos: Ann Betten Coming hSm Events HSM Honor Roll TomaTo LecTure/Hands-on WorksHop We thank the following volunteers (members as well as non-members) SATURDAY, MAY 6, 2017 who have supported the Society’s programs in recent months. 1:30 p.m. to 4 p.m. Cylburn Arboretum Greenhouse Classroom For the PPA/HSM Winter Seminar: Janet Draper, Mary Jo Sherrod, Craig LeHoullier, author of Epic Tomatoes. coordinators; Sally Barker, Helene Clapperton, Catherine Cook, Jennifer Members $25, Non-members $35. Forrence, Crystal Patterson and Paula Simon For the Plant Forum: Nancy Blois, Paula Campos, Helene Clapperton, annuaL Garden Tour Catherine Cook, Jennifer Forrence, Michael O’Rourke, Nancy Raskin, Mary SUNDAY, JUNE 4, 2017 Jo Sherrod, Lenel Srochi-Meyerhoff, Donna Watts and Bill Yonkers; and 10 a.m. -

Adenostoma Fasciculatum Profile to Postv2.Xlsx
I. SPECIES Adenostoma fasciculatum Hooker & Arnott NRCS CODE: ADFA Family: Rosaceae A. f. var. obtusifolium, Ron A. f. var. fasciculatum., Riverside Co., A. Montalvo, RCRCD Vanderhoff (Creative Order: Rosales Commons CC) Subclass: Rosidae Class: Magnoliopsida A. Subspecific taxa 1. Adenostoma fasciculatum var. fasciculatum Hook. & Arn. 1. ADFAF 2. A. f. var. obusifolium S. Watson 2. ADFAO 3. A. f. var. prostratum Dunkle 3. (no NRCS code) B. Synonyms 1. A. f. var. densifolium Eastw. 2. A. brevifolium Nutt. 3. none. Formerly included as part of A. f. var. f. C. Common name 1. chamise, common chamise, California greasewood, greasewood, chamiso (Painter 2016) 2. San Diego chamise (Calflora 2016) 3. prostrate chamise (Calflora 2016) Phylogenetic studies using molecular sequence data placedAdenostoma closest to Chamaebatiaria and D. Taxonomic relationships Sorbaria (Morgan et al. 1994, Potter et al. 2007) and suggest tentative placement in subfamily Spiraeoideae, tribe Sorbarieae (Potter et al. 2007). E. Related taxa in region Adenostoma sparsifolium Torrey, known as ribbon-wood or red-shanks is the only other species of Adenostoma in California. It is a much taller, erect to spreading shrub of chaparral vegetation, often 2–6 m tall and has a more restricted distribution than A. fasciculatum. It occurs from San Luis Obispo Co. south into Baja California. Red-shanks produces longer, linear leaves on slender long shoots rather than having leaves clustered on short shoots (lacks "fascicled" leaves). Its bark is cinnamon-colored and in papery layers that sheds in long ribbons. F. Taxonomic issues The Jepson eFlora and the FNA recognize A. f. var. prostratum but the taxon is not recognized by USDA PLANTS (2016). -

Oakmont Do Not Plant List
Oakmont Do Not Plant List Common Name Botanical Name Common Name Botanical Name Trees Shrubs/Vines Acacia Acacia spp. Bamboo ALL genera Arborvitae Thuja spp. Bluebeard Caryopteris spp. Australian tea tree Leptospermum Broom ALL genera laevigatum California buckwheat Eriogonum Black walnut Juglans nigra fasciculatum California bay Umbellularia Chamise or greasewood Adenostoma californica fasciculatum California pepper tree Schinus molle Chaparral pea Pickeringia montana Cedar Cedrus spp. Coyote brush Baccharis spp. Cypress Cupressus spp. Evergreen huckleberry Vaccinium ovatum Eucalyptus Eucalyptus spp. Gas plant Dictamus albus False cypress Chamaecyparis spp. Gorse Ulex europaeus Fir Abies spp. Honeysuckle Lonicera japonica ‘Halliana’ Giant chinquapin Chrysolepis chrysophylla Hopbush or hopseed Dodonaea viscosa bush Hemlock Tsuga spp. Juniper Juniperus spp. Honeylocust Gleditsia triacanthos Manzanita Arctostaphylos spp. Juniper Juniperus spp. (Ground cover variety okay) Leyland cypress Cupressus x leyandii (used as a hedge) New Zealand tea tree Leptospermum scoparium Palm ALL genera Rosemary Rosmarinus spp. Paperbark tree Melaleuca spp. Sagebrush Artemesia californica Pine Pinus spp. Scrub oak Quercus berberidifolia Spruce Picea spp. Grevillea Grevillea noellii Sweet gum Liquidambar Yew Taxus spp. (Also a styraciflua tree) Tamarisk Tamarix spp. Tan Oak Notholithocarpus densiflorus Tree of heaven Ailanthus altissima (Fourth Revision – 8/19/2018) (1/31/2021- Title and Spelling Corrections only) Common Name Botanical Name Grasses Fountain grass Pennisetum spp. Maiden grass Miscanthus sinensis Pampas grass Cortadaria selloana Ground Cover Big leaf periwinkle Vinca major Ivy Hedera spp. Juniper Juniperus spp. Mulch Woodchips and bark are not allowed in the 0–5- foot defensible space. The mulch types listed below are not allowed anywhere on residential property. Gorilla-hair Finely shredded redwood/cedar Rubber (Fourth Revision – 8/19/2018) (1/31/2021- Title and Spelling Corrections only) . -

Paper Version of Palos Verdes
Selected Plants Native to Palos Verdes Peninsula (C.M. Rodrigue, 07/26/11) http://www.csulb.edu/geography/PV/ Succulents (plants with fleshy, often liquid-saturated leaves and/or stems. These features can be found in a variety of life forms, including annual herbaceous plants, vines, shrubs, and trees, as well as cacti) Herbaceous plants (non-woody, though there may be a woody caudex or basal stem and root -- annual growth dies back each year, resprouting in perennial or biennial plants, or the plant dies and is replaced by a new generation each year in the case of annual plants) Extremely tiny plant. Stems only about 2-6 cm tall, occasionally as much as 10 cm, leaves only 1-3 mm long (can get up to 6 mm long), fleshy, found at the plant's base or on the stems, shape generally ovate (egg-shaped), may have a blunt rounded end or a fine acute tip. The leaves are arranged oppositely, not alternately. The plant is green when new but ages to red or pink. Tiny flower (0.5- 2 mm) borne in leaf axils, usually just one per leaf pair on a pedicel (floral stem) less than 6 mm long. Two or 3 petals and 3 or 4 sepals. Flowers February to May. Annual herb. Found in open areas, in rocky nooks and crannies, and sometimes in vernal ponds (temporary pools that form after a rain and then slowly evaporate). Crassula connata (Crassulaceae): pygmy stonecrop or pygmy-weed or sand pygmyweed Leaves converted into scales along stems, which are arranged alternately and overlap. -

Additional Details on Select Pollinator Plants Sugar Bush (Rhus Ovata
Additional Details on Select Pollinator Plants Sugar Bush (Rhus ovata) • Native to dry canyons/slopes of California • Flower colors are white and pink • Blooms during spring, winter • When established, needs only occasional watering and is easy to take care of • Prefers low moisture, is drought tolerant • Attracts butterflies and insects, bird friendly • Often used for firescaping (surrounding area with fire-resistant plants to create barrier) and slope stabilization (minimizing erosion) • Grows best in south facing chaparral slopes and hot/dry environments Pomegranate Trees • Pomegranate trees (Punica granatum) maximum height is 30 feet tall in ideal conditions, but commonly grow 12 to 16 feet tall as a round shrub or small tree. • The fruit is typically in season from September to February. • Prefer full sun exposure. • Once established, pomegranates can take considerable drought, but for good fruit production they must be irrigated Toyon Berry • Often grow to about 8 feet tall. • Its leaves are evergreen, alternate, sharply toothed, and are 5 cm in length and 2 cm wide. • The flowers are visited by butterflies and other insects, and have a mild, hawthorn-like scent. • They like sun or part shade, though they tend to do better in part shade in the southern, drier part of their geographic range. Canyon Sunflower (Venegasia carpesioides) • A great choice for a dry shady part of the garden as it tolerates full shade or part shade/sun. • Its yellow flowers are nearly always blooming. • They are fairly drought tolerant and they require little or no care and it’s tolerant of a variety of different garden soils. -

Rhus Integrifolia (Nutt.) Rothr., LEMONADEBERRY. Shrub Or Small
Rhus integrifolia (Nutt.) Rothr., LEMONADEBERRY. Shrub or small tree, evergreen, sclerophyllous, highly branched with dense canopy, in range 100–600 cm tall; gynodioecious; shoots in full sun with mostly ascending to erect leaves, in early spring forming new shoots from axillary buds below inflorescences, strongly aromatic like wintergreen (Gaultheria) when cut or crushed (having resin ducts with terpenes). Stems: cylindric, limber, when young red and brown with grayish cast from pubescence and scaly waxy plates; producing colorless resin in bark ducts, in ×-section the ducts elliptic, 16–18 arranged in ring; twig bark dull gray, trunk bark with thick, gray flakes; trunk to 40 cm diameter in arborescent forms. Leaves: helically alternate, simple, petiolate, without stipules; petiole 4–12(–15) mm long, reddish, sparsely pubescent and waxy with ± scaly plates; blade broadly elliptic to elliptic-ovate, (15–)25–70 × (10–)20–45 mm, leathery, flat or ± cupped to underside, broadly tapered to round at base, entire or coarsely serrate with shallow teeth and wavy on margins, ± transparent light pink or green at edge, ± obtuse to broadly acute at tip, pinnately veined with principal veins slightly raised on both surfaces, glabrous or with hairs and ± scaly waxy plates along lower midrib, satiny to glaucous, upper surface dark green, lower surface yellow-green. Inflorescence: panicle of spikes having either pistillate flowers or bisexual flowers (separate individuals), panicle 20–70 × 20–50 mm, compact spikes on primary to tertiary branches, many-flowered, -

1 Final Report Saratoga Horticultural Research Endowment 2013-2015
Final Report Saratoga Horticultural Research Endowment 2013-2015 Ornamental plant trials for the new California landscape: Evaluating industry introductions for sustainable characteristics on reduced water Principal Investigator: Karrie Reid, UCCE Environmental Horticulture Advisor, San Joaquin County 2101 E. Earhart Ave., Ste 200 Stockton, CA 95206-3949 (209) 953-6109 office (209) 953-6128 fax [email protected] Co-Investigators: David W. Fujino, Ph.D., Director, California Center for Urban Horticulture, UC Davis Lorence (Loren) R. Oki, Ph.D., CE Specialist, Dept. of Plant Sciences, UC Davis Jared Sisneroz, Staff Research Associate, Dept. of Plant Sciences, UC Davis Executive Summary In these trials 16 perennial landscape plant species, (9 new cultivars and 7 underutilized species/cultivars), were evaluated for overall performance on a range of reduced irrigation levels in clay loam soil in the hot interior Central Valley of California. All plants were grown in- ground for 2 years; 12 of the species in full sun and 4 under 50% shade. Planting in October 2013 was followed by an establishment period of irrigation at 80%-100% of reference evapotranspiration (ET0) and 25% management allowable depletion through April 2015. Plants were then subjected to 1 of 4 different levels of reduced irrigation at 20%, 40%, 60%, or 80% of ET0 during the dry season through the first week of October 2015. During the deficit irrigation season they were evaluated across treatments for growth, health and vigor, overall appearance, flowering, pest tolerance, and disease resistance. From these assessments, irrigation recommendations are made for their use in the landscape. Introduction Plant performance trials are a critical step in the introduction and promotion of new or unfamiliar ornamental plants. -

Pyrophytic Plants Vs. Fire Resistant Plants!
UNIVERSITYOF CALIFORNIA COOPERATIVE EXTENSION run0rilfficv$,flmnastsT,{NT PIJTNI$ Ray Moritz, Fire Ecologistanil TcchnicalAdvisor to FireSafe Madn; and. Pavel Svihra, Horticulture Aduisor,Main and SonomaCounties "White house" and its garden (trace with a dashed line) is a spectacular demonstration of defensible space.Wildfire with cata- clysmic energy swiftly burned adjacent houses and vegetation, yet it died down after reaching the frontier of fire resistant garden. PYROPHYTES They may have loose or papery bark. Plantsthat ignite readily and burn intensely(pyro- . These plants flame (not smolder) when phates)typically sharecertain characteristics: preheated and ignited with a match. They are usually blade-leaf or needle-leaf evergreens. FLAMMABILITY & CONDITION o They have stiff, woody, small or finer lacey The condition of the plant is as importantas its spe- leav"es. cies.Even some"pyrophytic" speciescan be quite fire-resistantif properly maintained. Their leaves and wood usuallv contain volatile waxes, fats, terpenes or oils (drushed leaves have strong od6rs). Depending on its growth form and accessto water, the samespecies may be fire resistantin one envi- ronment and pyrophytic in another.Water-stressed o Their sap $ usually gummy' resinous and has a strong odor. plants that are in poor condition are more pyrophytic. They usually contain plentiful fine, twiggy, A pyrophytic speciesbecomes explosively flamma- drv or dead materials. ble when poorly maintained. South-facing slopes, windy areas,sites with poor soils and urban land- . They may have pubescent (hair covered) leaves. scapesare more stressfulfor plants. (cmcer-related), mce Po1icymayboaddressedtotheAfimativeAc!ionDirector,Unive.sityofcali1bmia,AgriculoreadNafralResources,llll Fire ResistantPlants PYROPTTYTICVBGETATION TYPES : Grasses: Any cured (dry) grass.Nonirrigated, bracken and sword ferns.