Development and Characterization of an Immunologically Humanized And
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Mammalian Anatomy: a Fetal Pig Dissection
Mammalian Anatomy: A Fetal Pig Dissection Background: Introduce the natural history of this animal, using its scientific name, and the reason for its dissection. Include a brief review of all of the levels of classification for this animal and a few features typical at each level, be sure to include chordate and vertebrate features as well as the characteristics specific to mammals. Purpose: Dissect a fetal pig to observe characteristics of placental mammalian anatomy and physiology and contrast them to that of humans. Materials: List appropriately Procedure: 1. Survey characteristics of vertebrate body systems in comparison to invertebrate systems. 2. Review dissection tools, procedures, safety precautions. 3. Follow the dissection as given in class (cite the handout). Results: A clear photo from each section neatly and properly identified and labeled should be included. Try to use the fewest number of pictures as possible, not a photo for each organ…brilliant and concise! Analysis: Use the purpose statement as the basis for the analysis and conclusion. There are three areas of interest and there should be a well developed discussion of each with examples from the dissection and references to the results. Conclusion: Give clear and concise statement of the actual results that addresses the purposes of the lab. Limit to a few sentences. Mammalian Anatomy: A Fetal Pig Dissection Introduction: Eutherian, or placental, mammals share many physical traits such as body hair, mammary glands and nipples, a four chambered hear, specialized teeth, a diaphragm and specialized digestive, respiratory, circulatory, excretory and reproductive systems. As we study this fetal pig’s anatomy we will learn more about these common traits and therefore more about our human body systems. -
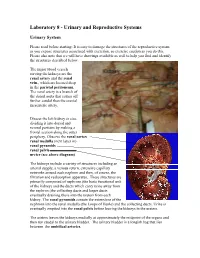
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Fetal Pig Dissection Lab with Detailed Images
Fetal Pig Dissection Lab with Detailed Images Beth Cantwell and Laurel Rodgers Shenandoah University, 1460 University Drive, Winchester, VA 22601 [email protected], [email protected] Abstract This lab provides students with detailed dissection instructions of the fetal pig, including the digestive, respiratory, cardiac, urinary, and reproductive systems. Color pictures are provided for each step to help students ensure that they are removing the correct tissues and making the correct incisions. Information is provided throughout the lab about the function of each organ students observe. This lab was written for a non-majors, general education course. However, we have provided additional materials in the appendix in order for instructors to expand the lab for a Biology majors course. Keywords: Anatomy, organ systems, fetal pig dissection Introduction The fetal pig is frequently used during anatomy units in both majors and non-majors courses because the pig has a similar anatomy to humans, is reasonably priced, and is readably available from multiple sources. In our non-majors Biology course, Biology in Society, we use the fetal pig to introduce our students to basic anatomy and the function of some major biological systems in the human. We wrote this lab because we have struggled to find a fetal pig dissection that contained both sufficient instructions and information for our course. Detailed instructions allow students to make the appropriate incisions and observe organs with minimal assistance from the instructor. From a classroom management perspective, this is important because it allows the lab instructor to circulate quickly within a group of up to twenty students. -

Fetal Pig Dissection
FETAL PIG DISSECTION In this lab exercise you will open the abdominal-pelvic and thoracic cavities of a fetal pig and identify its major organs. Remember you are dissecting not butchering. The goal is for you to identify all of the structures described herein via a careful and thorough dissection. Do not remove any organs. Once an organ or structure has been identified be sure to compare it to the corresponding one in the human torso. Most structures will appear to be similar but there are a several important exceptions. DIRECTIONAL TERMS Ventral Toward the belly Lateral Away from midline Dorsal Toward the back or spine Anterior Relative to forward movement Superficial At the surface Posterior Opposite of anterior Deep Toward the interior Proximal Toward center or attachment point Medial Toward midline or midsagittal plane Distal Away from center or attachment DETERMINING THE SEX OF YOUR SPECIMEN Males are identified by the presence of a urogenital opening or preputial orifice just posterior to the umbilical cord as shown in the left panel below. Depending on the age of the pig it may be possible to see or feel the penis as it passes towards this opening. Males also possess a pair of scrotal swellings at the posterior end that can help in identification. In contrast to males, the urogenital opening in females is immediately ventral to or below the tail and anus. This opening is also marked by a fleshy tubercle called the genital papilla as shown in the right panel below. The presence (or absence) of a genital papilla is probably the most straight-forward way for you to determine the sex of your specimen. -

Fetal Pig Visual Dissection Guide Illustrated by Leah Hofgesang
Fetal Pig Visual Dissection Guide Illustrated by Leah Hofgesang WARD470156-776 © 2015 Ward’s Science All Rights Reserved Orientation orsal D Cranial Caudal Anterior erse plane Posterior v ans r T Frontal plane Sagittal plane al r t n e V Illustrated by Leah Hofgesang 1 © 2015 Ward’s Science All Rights Reserved 1 Incisions Gender Key Male Female Both 4 3 Umbilical vein Umbilical arteries 2 1 © 2015 Ward’s Science All Rights Reserved 2 Illustrated by Leah Hofgesang Internal Organs Submaxillary gland Larynx Common carotid artery Internal jugular vein External jugular vein Trachea Thymus gland Left atrium Right atrium Lungs Right ventricle Diaphram Liver Spleen Large intestine Small intestine Umbilical cord Bladder Rectum Illustrated by Leah Hofgesang 3 © 2015 Ward’s Science All Rights Reserved Abdominal Procedure View the Gallbladder and Stomach Grasp inferior edge of the liver and fip superiorly to view organs dorsal of the liver. Esophagus Liver Gallbladder Stomach Duodenum Large Jejunum intestine Ileum Small intestine Rectum Gallbladder Stomach © 2015 Ward’s Science All Rights Reserved 4 Illustrated by Leah Hofgesang Internal Organs Underneath Liver Liver (left lobe) Liver (right lobe) Gall bladder Spleen Stomach Small intestine Large intestine Umbilical cord Bladder Illustrated by Leah Hofgesang 5 © 2015 Ward’s Science All Rights Reserved Abdominal Procedure View the Pancreas and Kidneys Carefully remove the liver (optional). Grasp the small intestines and carefully pull laterally to reveal the pancreas and kidneys. Notes Small intestine -

Beyond Dissection: Innovative Tools for Biology Education. Ethical
DOCUMENT RESUME ED 438 188 SE 063 218 AUTHOR Larson, Sandra, Ed. TITLE Beyond Dissection: Innovative Tools for Biology Education. INSTITUTION Ethical Science Education Coalition, Boston, MA. PUB DATE 1998-02-00 NOTE 88p. AVAILABLE FROM NEAVS, 333 Washington St., Suite 850, Boston, MA 02108-5100 ($2). Tel: 617-523-6020; Web site: http://www.neays.org/esec.html. *B TYPE Reference Materials Directories/Catalogs (132) EDRS PRICE MF01/PC04 Plus Postage. DESCRIPTORS *Anatomy; Audiovisual Aids; *Biology; Charts; Computer Software; Diagrams; *Dissection; Elementary Secondary Education; Higher Education; *Instructional Materials; Manipulative Materials; Multimedia Materials; Physiology; Science Education; Zoology ABSTRACT This catalog lists resources available for classroom use in teaching about anatomy and physiology which are alternatives to dissection. The entries are provided under three main categories:(1) Whole Animal Dissection/Vivisection;(2) Animal Organ or System Anatomy and Physiology; and (3) Other, including animal behavior, biOtechnology, embryology, innovative teaching tools, school hatching projects, and wonder and respect. EaCh resource listing contains the product title, supplier/publisher contact information, description, price, and age range. Listings include models, slides, videos, computer programs, cards, charts, and other materials.(WRM) Reproductions supplied by EDRS are the best that can be made from the original document. a a I I PERMISSION TO REPRODUCE AND DISSEMINATE THIS MATERIAL HAS BEEN GRANTED Y .1_ I 0 TO THE -

12Chapter the Fetal Pig Dissection
Chapter12 The Fetal Pig Dissection Objectives 1. Compare and contrast the anatomy of human and pig organ systems. 2. Identify the muscles of the pig back, shoulder, neck, chest, abdomen, forelimb and hindlimb. 3. Identify the major nerves of the brachial and sacral plexuses. 4. Dissect and examine the spinal cord. 5. Locate and identify the major glands of the endocrine system, and associate function with the hormone produced within each gland. 6. Identify the major arteries and veins of the pig circulatory system. 7. Identify and locate the major organs of the lymphatic, respiratory, digestive and reproductive systems. Shutterstock 490 Chapter 12 Essentials of Anatomy and Physiology Lab II ach section of this lab will describe the steps required to view structures found in each of the major organ systems. It is important to keep in mind that the Eterminology used to describe the position and location of body parts in four- legged animals, like the pig, is slightly different than what is used for humans. First, the anatomical position of a four-legged animal occurs when all legs are on the ground. Superior refers to the back (or dorsal) surface and inferior indicates the belly (or ventral) surface. Cephalic or anterior refers to the front (towards the head), and caudal or poste- rior refers to the rear structures. Before beginning this lab exercise, consult with your laboratory instructor as to which of the below activities you will complete as it is not always necessary or desired to complete them all. 12.1 Muscular System of the Pig As an introduction into the pig muscular system, please note that some muscles found in four-legged animals are lacking in humans, and some muscles are fused in humans but are actually separate muscles in the four-legged animals. -

Development of the Pig Placenta*
Development of the pig placenta* J.L. Vallet, J.R. Miles and B.A. Freking USDA, ARS, U.S. Meat Animal Research Center, P. 0. Box 166, State Spur 18D, Clay Center, Nebraska 68933, USA Placental insufficiency results in fetal loss, low birth weight, stillbirth, preweaning mortality and poor growth. Placental development begins at conceptus elongation, which is a primary factor controlling the size of the placenta. After elongation, the allantois develops outward from the embryo to establish the al lantochorion, which defines the size of the functional placenta. During implantation, chorionic trophoblasts adhere to endometrial epithelial cells. Placental structures known as areolae develop at the openings of the endometrial glands and take up endometrial gland secreted products (histotrophe). Between day 30 and 35 of gestation, the adhered trophoblast-endometrial epithelial bi layer undergoes microscopic folding. Fetal and maternal capillaries develop adjacent to the bilayer and blood flows are arranged in a cross-countercurrent manner. Except for nutrients secreted by the glands, nutrient exchange takes place between these capillaries within these folds. By day 85, the folds deepen and become more complex, increasing surface area. The epithelial bilayer thins and capillaries indent the plane of each layer (but do not penetrate), reducing distance between capillaries. The folded bilayer is surrounded by endometrial stroma on the maternal side and placental stroma on the fetal side. The fetal-placental stroma is partially composed of glycosaminoglycans, the most abundant being hyaluronan and heparan sulfate. Changes in both hyaluronoglucosaminidase and heparanase during placental development suggest that these enzymes play a role in placental development. -

Fetal Pig Dissection What Do You Think Humans Have in Common with the Pig?
Fetal Pig Dissection What do you think humans have in common with the pig? https://ferrebeekeeper.files.wordpress.com/2014/03/farmer-clip-art-4.gif http://www.clipartpanda.com/categories/pig-in-mud-cartoon Humans and Pigs may be closer than you think! Both are mammals We share common body systems The anatomy of the pig is close to that of humans The fetal pigs will tell us more about our own bodies and give us a way to explore! http://www.fanpop.com/clubs/human-anatomy/images/10358267/title/human-anatomy-photo http://www.biologycorner.com/pig/fetal_pig02.jpg SAFETY FIRST! ❖ NEVER point sharp ❖ Wash hand at the objects at yourself or end of each class others period ❖ Always cut/make ❖ Don’t remove any incisions away form specimens from the yourself and others classroom ❖ No horseplay in the ❖ Properly dispose of lab any materials ❖ Properly mount ❖ Clean up area specimens to the dissection pan ❑ Descriptive words are used to describe “where” on an animal. ❑ Like using North, South, East, or West for locations on a map. Dorsal -- toward the back Ventral -- toward the front/belly Separated by the frontal plane V Cranial -- toward the head Rostral -- toward the nose/beak Caudal -- toward the tail Separated by the transverse plane Medial – directed toward the midline (sagittal plane) Lateral -- directed away from the midline (sagittal plane) Sagittal Plane Proximal -- located close to the sagittal line of the body. Distal -- located away from the sagittal line of the body External Anatomy ❖ Skin ❖ Nose ❖ Tongue ❖ Eyelids ❖ External Ear ❖ Digits ❖ Umbilical Cord ❖ Identify the Sex ❖ 2 umbilical arteries ❖ Male – Scrotal Sac (ventral to anus) ❖ Umbilical vein and Urogenital Opening ❖ Teats ❖ Female – Urogenital opening (ventral ❖ Anus to anus) and genital papilla DEMO SLIDE BOX 23 Commercial slide. -

FETAL PIG DISSECTION a LABORATORY GUIDE 8545D Fm I-Iv 6/26/02 3:56 PM Page Ii Mac62 Mac62:1253 GE: 8545D Fm I-Iv 6/26/02 3:56 PM Page Iii Mac62 Mac62:1253 GE
8545d_fm_i-iv 6/26/02 3:56 PM Page i mac62 mac62:1253_GE: FETAL PIG DISSECTION A LABORATORY GUIDE 8545d_fm_i-iv 6/26/02 3:56 PM Page ii mac62 mac62:1253_GE: 8545d_fm_i-iv 6/26/02 3:56 PM Page iii mac62 mac62:1253_GE: FETAL PIG DISSECTION A LABORATORY GUIDE CONNIE ALLEN VALERIE HARPER Edison Community College John Wiley & Sons, Inc. 8545d_fm_i-iv 6/26/02 3:48 PM Page iv mac62 mac62:1253_GE: Senior Editor Bonnie Roesch Associate Editor Mary O’Sullivan Production Editor Sandra Russell Senior Marketing Manager Clay Stone Senior Designer Kevin Murphy Book Designer Nancy Field Photo Manager Hilary Newman Illustration Editor Anna Melhorn Production Management Services TechBooks All photos provided courtesy of Dr. William Benyak. This book was typeset in 10/12 Times Roman by TechBooks, Inc. and printed and bound by Von Hoffmann Press. The cover was printed by Von Hoffmann Corporation. The paper in this book was manufactured by a mill whose forest management programs include sustained yield harvesting of its timberlands. Sustained yield harvesting principles ensure that the number of trees cut each year does not exceed the amount of new growth. This book is printed on acid-free paper. ∞ Copyright © 2003 by John Wiley & Sons, Inc. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying recording, scanning or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (508) 750-8400, fax (508) 750-4470. -
Fetal Pig Dissection Labs Dr
Fetal Pig Dissection Labs Dr. J. Lim Objective: In this exercise you will examine the organization of the many body systems studied this semester in the context of a single specimen, the fetal pig. Be sure to identify the major organs as you explore the extent of each system. As you encounter each structure, discuss its function and interactions with surrounding structures with your lab partners Recommendations: • Carefully follow the directions • Read the description of each incision and understand it prior to beginning • Each group should have a directions reader and a dissector • The rest of the group should follow along referring to the textbook or lecture notes with details on the structures being studied • Use the scissors for most incisions • All students handling the specimen must wear gloves Use the scalpel only when absolutely necessary Clean-up at the end of each dissection: • Dispose of lab discards in the provided receptacles • Place your pig into the plastic bag provided o Expel excess air from the bag and tie it shut o Write your group name and class time on the tag provided and attach it to the bag o Place the bags in the storage bin for your class • Return dissection instruments to their proper places in the set-up tray • Clean table tops with red bottled sanitizer • Wash hands before leaving class 1 FETAL PIG LAB ONE: Respiratory 1, Mouth, Pharynx & Thorax External Anatomy • Examine the fetal pig and locate the external features shown above. • Two rows of nipples of mammary glands are present on the ventral abdominal surface of both males and females. -

Anatomy of the Pig by Tori Lev Fetal Pig Dissection Umans and Fetal Pigs Share Very Similar Intermediate School
Anatomy of the Pig By Tori Lev Fetal Pig Dissection umans and fetal pigs share very similar intermediate school. Pigs are also important Hanatomy as they are both mammals. to study because recent research has found Fetal pigs are also a popular choice for that they are so similar to humans that dissections as they are a bi-product of the their heart tissues can be used for human pork industry, therefore as long as people transplants, after the tissue is treated retain their love for bacon, they will always to reduce potential rejection. For this be available in abundance. Furthermore, article, two nine inch pigs were dissected. fetal tissue is soft and easy to cut through, making it an ideal specimen, especially for Key Terms Dorsal - the back Ventral - the front (belly-side) Median - the middle/central Median plane - divides body into two similar halves Lateral - the side Anterior - the front (head-end) Posterior - the back (tail-end) Proximal - situated near the point of attachment on body Distal - farthest away from point of attachment on body Superficial - near the surface Deep - below the surface 1 | Anatomy of the Pig The Heart 9 he heart and lungs are both located 7 Tin a region of the pig known as the 8 thoracic cavity, which is separated from the digestive system by the diaphragm, a thin, sheet-like muscle. Between the lungs we 6 find the heart swaddled in the pericardial 1 sac. Here we can begin to see that the 2 heart is separated into four chambers - the right and left atrium as well as the right 3 and left ventricle.