Innate and Adaptive Immune Responses in the Lungs
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
IFM Innate Immunity Infographic
UNDERSTANDING INNATE IMMUNITY INTRODUCTION The immune system is comprised of two arms that work together to protect the body – the innate and adaptive immune systems. INNATE ADAPTIVE γδ T Cell Dendritic B Cell Cell Macrophage Antibodies Natural Killer Lymphocites Neutrophil T Cell CD4+ CD8+ T Cell T Cell TIME 6 hours 12 hours 1 week INNATE IMMUNITY ADAPTIVE IMMUNITY Innate immunity is the body’s first The adaptive, or acquired, immune line of immunological response system is activated when the innate and reacts quickly to anything that immune system is not able to fully should not be present. address a threat, but responses are slow, taking up to a week to fully respond. Pathogen evades the innate Dendritic immune system T Cell Cell Through antigen Pathogen presentation, the dendritic cell informs T cells of the pathogen, which informs Macrophage B cells B Cell B cells create antibodies against the pathogen Macrophages engulf and destroy Antibodies label invading pathogens pathogens for destruction Scientists estimate innate immunity comprises approximately: The adaptive immune system develops of the immune memory of pathogen exposures, so that 80% system B and T cells can respond quickly to eliminate repeat invaders. IMMUNE SYSTEM AND DISEASE If the immune system consistently under-responds or over-responds, serious diseases can result. CANCER INFLAMMATION Innate system is TOO ACTIVE Innate system NOT ACTIVE ENOUGH Cancers grow and spread when tumor Certain diseases trigger the innate cells evade detection by the immune immune system to unnecessarily system. The innate immune system is respond and cause excessive inflammation. responsible for detecting cancer cells and This type of chronic inflammation is signaling to the adaptive immune system associated with autoimmune and for the destruction of the cancer cells. -

Lung Anatomy
Lung anatomy Breathing Breathing is an automatic and usually subconscious process which is controlled by the brain. The brain will determine how much oxygen we require and how fast we need to breathe in order to supply our vital organs (brain, heart, kidneys, liver, stomach and bowel), as well as our muscles and joints, with enough oxygen to carry out our normal daily activities. In order for breathing to be effective we need to use our lungs, breathing muscles and blood system efficiently. This leaflet should help you to better understand the process of breathing and how we get the much needed oxygen into our bodies. The lungs You have two lungs, one in the right side and one in the left side of your chest. The right lung is bigger than the left due to the position of the heart (which is positioned in the left side of the chest). Source: Pulmonary Rehabilitation Reference No: 66354-1 Issue date: 13/2/20 Review date: 13/2/23 Page 1 of 5 Both lungs are covered by 2 thin layers of tissue called the pleura. The pleura stop the surface of the lungs rubbing together as we breathe in and out. The lungs are protected by the ribcage. The airways Within the lungs there is a vast network of airways (tubes) which help to transport the oxygen into the lungs and the carbon dioxide out. These tubes branch into smaller and smaller tubes the further they go into the lungs. Page 2 of 5 Trachea (windpipe): This tube connects your nose and mouth to your lungs. -

Innate Immunity in the Lung: How Epithelial Cells Fight Against
Copyright #ERSJournals Ltd 2004 EurRespir J 2004;23: 327– 333 EuropeanRespiratory Journal DOI: 10.1183/09031936.03.00098803 ISSN0903-1936 Printedin UK –allrights reserved REVIEW Innateimmunity in the lung: how epithelialcells ght against respiratorypathogens R. Bals*,P.S. Hiemstra # Innateimmunity in the lung: how epithelial cells ® ghtagainst respiratory pathogens *Deptof Internal Medicine, Division of R Bals, P S Hiemstra #ERS JournalsLtd 2004 PulmonaryMedicine, Hospital of the Uni- versityof Marburg, Philipps-University, ABSTRACT: Thehuman lung is exposed to a largenumber of airborne pathogens as a # resultof the daily inhalation of 10,000 litres of air Theobservation that respiratory Marburg,Germany; Deptof Pulmonology, LeidenUniversity Medical Center, Leiden, infectionsare nevertheless rare is testimony to the presence of anef®cient host defence TheNetherlands systemat the mucosal surface of the lung Theairway epithelium is strategically positioned at the interface with the Correspondence:P S Hiemstra,Dept of environment,and thus plays a keyrole in this host defence system Recognition Pulmonology,Leiden University Medical systemsemployed by airwayepithelial cells to respond to microbialexposure include the Center, P O Box9600, 2300 RC Leiden, The actionof the toll-like receptors Netherlands Theairway epithelium responds to such exposure by increasing its production of Fax:31 715266927 mediatorssuch as cytokines, chemokines and antimicrobial peptides Recent® ndings E-mail: p s hiemstra@lumc nl indicatethe importance of these peptides -

Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases
microorganisms Review Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases Juan Alberto Lira-Lucio 1 , Ramcés Falfán-Valencia 1 , Alejandra Ramírez-Venegas 2, Ivette Buendía-Roldán 3 , Jorge Rojas-Serrano 4 , Mayra Mejía 4 and Gloria Pérez-Rubio 1,* 1 HLA Laboratory, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] (J.A.L.-L.); [email protected] (R.F.-V.) 2 Tobacco Smoking and COPD Research Department, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] 3 Translational Research Laboratory on Aging and Pulmonary Fibrosis, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] 4 Interstitial Lung Disease and Rheumatology Unit, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] (J.R.-S.); [email protected] (M.M.) * Correspondence: [email protected]; Tel.: +52-55-5487-1700 (ext. 5152) Received: 11 June 2020; Accepted: 7 July 2020; Published: 16 July 2020 Abstract: The lung microbiome composition has critical implications in the regulation of innate and adaptive immune responses. Next-generation sequencing techniques have revolutionized the understanding of pulmonary physiology and pathology. Currently, it is clear that the lung is not a sterile place; therefore, the investigation of the participation of the pulmonary microbiome in the presentation, severity, and prognosis of multiple pathologies, such as asthma, chronic obstructive pulmonary disease, and interstitial lung diseases, contributes to a better understanding of the pathophysiology. Dysregulation of microbiota components in the microbiome–host interaction is associated with multiple lung pathologies, severity, and prognosis, making microbiome study a useful tool for the identification of potential therapeutic strategies. -

Qnas with Max D. Cooper and Jacques F. A. P. Miller
QNAS QnAswithMaxD.CooperandJacquesF.A.P.Miller QNAS Brian Doctrow, Science Writer Anyone who has ever contracted chicken pox can thank the adaptive immune system for future pro- tection against the disease. It is also thanks to this system that vaccines prevent diseases. The adaptive immune system provides organisms with a memory of past infections, enabling the body to quickly kill returning infections before they can do significant damage. Immunologists Jacques F. A. P. Miller and Max D. Cooper determined that adaptive immunity requires 2 distinct cell types that perform comple- mentary functions. Miller’s findings, published in the early 1960s in Lancet (1) and Proceedings of the Royal Society (2), showed that the ability to distinguish one’s own cells from foreign cells, a key feature of the adap- tive immune system, depends on lymphocytes, now known as T cells, matured in an organ called the thy- mus. Subsequently, Cooper reported in Nature (3) that Max Dale Cooper. Image courtesy of Georgia Research antibody production depends on a separate set of Alliance/Billy Howard. lymphocytes, dubbed B cells. The division of labor between T and B cells is a fundamental organizing principle of the adaptive immune system, the discov- did cancer research. I started working on leukemia and ery of which laid the groundwork for modern immu- this gave me an interest in lymphocytes. nology and made possible many subsequent medical advances, including monoclonal antibody produc- Cooper: I became interested through patients that I tion, vaccine development, and checkpoint inhibi- was taking care of: Children that had deficient immune tion therapies for cancer. -

Manatee Anatomy and Physiology
Manatee Anatomy and Physiology Grade level: Elementary 5 Subject Area: Biology, Anatomy and Physiology, Marine Biology Duration: Teach: 15 minutes, Activity: 20 minutes, Discussion: 20 minutes. Setting: Classroom Next Generation Sunshine State Standards: Reading: LAFS.5.RI Reading standards for Information text Cluster 3 LAFS.5.RI.3 Writing: LA.B.2.1, LA.B.1.2 Speaking and Listening: Cluster 2 LAFS.5.SL.Z Presentation of Knowledge and Ideas Science: Body of Knowledge Life Science: SC.5.L.14, SC.5.L.15, SC.5.L.17, Body of Knowledge Nature of Science Supporting Idea 1: SC.5.N.1 Practice of Science, Supporting Idea 2:SC.5.N.2 Characteristics of Scientific Knowledge Earth Systems and Patterns: 7SC.E.E.7 Statewide Science Assessment Prompt: How might humans help manatees survive? Objectives: Students will learn about manatee bodies and explain some anatomical and physiological differences between manatees, humans and other animals. Materials: Handouts of basic manatee anatomy, dolphin anatomy & human anatomy, crayons or markers, coloring direction sheet, question worksheet, Quiz sheet Vocabulary: Mammal, endangered species, habitat, conservation, vibrissae, nares, blowhole, flipper, herbivore, omnivore, carnivore. Background/Preparation: Handouts of manatee, dolphin, and human anatomy. Fact sheets comparing and contrasting specific and unique anatomical aspects of each species. Basic manatee fact sheet highlighting personality, limited habitat, endangered status and conservation efforts. Teachers can review the manatee fact sheets, and select points of interest they would most like to incorporate into a lesson. This activity may fit best into the week where the human anatomy lessons are addressed. Teachers can present the information via traditional lecture, group discussion, question and answer session, or doing the coloring activity as the lesson points are addressed, etc. -

The Fifth Pulmonary Vein
CASE REPORT Anatomy Journal of Africa. 2016. Vol 5 (2): 704 - 706 The Fifth Pulmonary vein Hilkiah Kinfemichael, Asrat Dawit Correspondence to Hilkiah Kinfemichael, Myungsung Medical College, Addis Ababa, Ethiopia PO Box 14972 Email : [email protected]. ABSTRACT A cadaver in Myungsung Medical College (MMC) had a 3rd pulmonary vein originating from the middle lobe of the right lung. Such anatomical variations are very rare. People with this variation have a total of five pulmonary veins entering left atrium. It has clinical implications especially for thoracic surgeons and radiologists during radiofrequency ablations, lobectomies, valve replacements, pulmonary vein catheterizations, video-assisted thoracic surgery (VATS) and others. Key Words: Anatomy, Variations, Pulmonary veins. INTRODUCTION Two pulmonary veins usually drain oxygenated upper lobe, is formed by the union of blood from each lung to left atrium of the apicoposterior, anterior and lingular veins. The heart. The lobular tributaries lie mainly in the inferior left pulmonary vein, which drains the interlobular septa and two pulmonary veins lower lobe, is formed by the hilar union of two from each lung enter left atrium through two veins, superior and common basal veins. The separate pulmonary ostia on either side. On the right and left pulmonary veins perforate the right lung veins from the apical, anterior and fibrous pericardium and open separately in the posterior part of upper lobe unite with a middle posterosuperior aspect of the left atrium lobar vein, which is formed by lateral and (Standring et al., 2008). This anatomy could medial tributaries, and form the superior right show variations, with more veins draining pulmonary vein (Standring, 2008). -
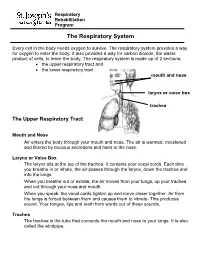
The Respiratory System
Respiratory Rehabilitation Program The Respiratory System Every cell in the body needs oxygen to survive. The respiratory system provides a way for oxygen to enter the body. It also provides a way for carbon dioxide, the waste product of cells, to leave the body. The respiratory system is made up of 2 sections: the upper respiratory tract and the lower respiratory tract mouth and nose larynx or voice box trachea The Upper Respiratory Tract Mouth and Nose Air enters the body through your mouth and nose. The air is warmed, moistened and filtered by mucous secretions and hairs in the nose. Larynx or Voice Box The larynx sits at the top of the trachea. It contains your vocal cords. Each time you breathe in or inhale, the air passes through the larynx, down the trachea and into the lungs. When you breathe out or exhale, the air moves from your lungs, up your trachea and out through your nose and mouth. When you speak, the vocal cords tighten up and move closer together. Air from the lungs is forced between them and causes them to vibrate. This produces sound. Your tongue, lips and teeth form words out of these sounds. Trachea The trachea is the tube that connects the mouth and nose to your lungs. It is also called the windpipe. The Lower Respiratory Tract Inside Lungs Outside Lungs bronchial tubes alveoli diaphragm (muscle) Bronchial Tubes The trachea splits into 2 bronchial tubes in your lungs. These are called the left bronchus and right bronchus. The bronchus tubes keep branching off into smaller and smaller tubes called bronchi. -

Lung Lobe Segmentation Based on Lung Fissure Surface Classification
algorithms Article Lung Lobe Segmentation Based on Lung Fissure Surface Classification Using a Point Cloud Region Growing Approach Xin Chen 1, Hong Zhao 2 and Ping Zhou 3,* 1 College of Electrical and Information Engineering, Hunan University, Changsha 410082, China; [email protected] 2 College of Aerospace Science and Engineering, National University of Defense Technology, Changsha 410003, China; [email protected] 3 College of Biology, Hunan University, Changsha 410082, China * Correspondence: [email protected] Received: 2 September 2020; Accepted: 13 October 2020; Published: 15 October 2020 Abstract: In anatomy, the lung can be divided by lung fissures into several pulmonary lobe units with specific functions. Identifying the lung lobes and the distribution of various diseases among different lung lobes from CT images is important for disease diagnosis and tracking after recovery. In order to solve the problems of low tubular structure segmentation accuracy and long algorithm time in segmenting lung lobes based on lung anatomical structure information, we propose a segmentation algorithm based on lung fissure surface classification using a point cloud region growing approach. We cluster the pulmonary fissures, transformed into point cloud data, according to the differences in the pulmonary fissure surface normal vector and curvature estimated by principal component analysis. Then, a multistage spline surface fitting method is used to fill and expand the lung fissure surface to realize the lung lobe segmentation. The proposed approach was qualitatively and quantitatively evaluated on a public dataset from Lobe and Lung Analysis 2011 (LOLA11), and obtained an overall score of 0.84. Although our approach achieved a slightly lower overall score compared to the deep learning based methods (LobeNet_V2 and V-net), the inter-lobe boundaries from our approach were more accurate for the CT images with visible lung fissures. -

The Role of Microbiota in the Pathogenesis of Esophageal Adenocarcinoma
biology Review The Role of Microbiota in the Pathogenesis of Esophageal Adenocarcinoma Megan R. Gillespie 1, Vikrant Rai 2 , Swati Agrawal 3 and Kalyana C. Nandipati 3,* 1 School of Medicine, Creighton University, 2500 California Plaza, Omaha, NE 68178, USA; [email protected] 2 Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA; [email protected] 3 Department of Surgery, Creighton University School of Medicine, 2500 California Plaza, Omaha, NE 68178, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-402-280-5292 Simple Summary: Esophageal adenocarcinoma has a poor 5-year survival rate and is among the highest mortality cancers. Changes in the esophageal microbiome have been associated with cancer pathogenesis; however, the molecular mechanism remains obscure. This review article critically analyzes the molecular mechanisms through which microbiota may mediate the development and progression of esophageal adenocarcinoma and its precursors-gastroesophageal reflux disease and Barrett’s esophagus. It summarizes changes in esophageal microbiome composition in normal and pathologic states and subsequently discusses the role of altered microbiota in disease progression. The potential role of esophageal microbiota in protecting against the development of esophageal adenocarcinoma is also discussed. By doing so, this article highlights specific directions for future research developing microbiome-mediated therapeutics for esophageal adenocarcinoma. Abstract: Esophageal adenocarcinoma (EAC) is associated with poor overall five-year survival. The Citation: Gillespie, M.R.; Rai, V.; incidence of esophageal cancer is on the rise, especially in Western societies, and the pathophysiologic Agrawal, S.; Nandipati, K.C. The Role of Microbiota in the Pathogenesis of mechanisms by which EAC develops are of extreme interest. -

Human Autoimmunity and Associated Diseases
Human Autoimmunity and Associated Diseases Human Autoimmunity and Associated Diseases Edited by Kenan Demir and Selim Görgün Human Autoimmunity and Associated Diseases Edited by Kenan Demir and Selim Görgün This book first published 2021 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2021 by Kenan Demir and Selim Görgün and contributors All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-5275-6910-1 ISBN (13): 978-1-5275-6910-2 TABLE OF CONTENTS Preface ...................................................................................................... viii Chapter One ................................................................................................. 1 Introduction to the Immune System Kemal Bilgin Chapter Two .............................................................................................. 10 Immune System Embryology Rümeysa Göç Chapter Three ............................................................................................. 18 Immune System Histology Filiz Yılmaz Chapter Four .............................................................................................. 36 Tolerance Mechanisms and Autoimmunity -

Immunology 101
Immunology 101 Justin Kline, M.D. Assistant Professor of Medicine Section of Hematology/Oncology Committee on Immunology University of Chicago Medicine Disclosures • I served as a consultant on Advisory Boards for Merck and Seattle Genetics. • I will discuss non-FDA-approved therapies for cancer 2 Outline • Innate and adaptive immune systems – brief intro • How immune responses against cancer are generated • Cancer antigens in the era of cancer exome sequencing • Dendritic cells • T cells • Cancer immune evasion • Cancer immunotherapies – brief intro 3 The immune system • Evolved to provide protection against invasive pathogens • Consists of a variety of cells and proteins whose purpose is to generate immune responses against micro-organisms • The immune system is “educated” to attack foreign invaders, but at the same time, leave healthy, self-tissues unharmed • The immune system can sometimes recognize and kill cancer cells • 2 main branches • Innate immune system – Initial responders • Adaptive immune system – Tailored attack 4 The immune system – a division of labor Innate immune system • Initial recognition of non-self (i.e. infection, cancer) • Comprised of cells (granulocytes, monocytes, dendritic cells and NK cells) and proteins (complement) • Recognizes non-self via receptors that “see” microbial structures (cell wall components, DNA, RNA) • Pattern recognition receptors (PRRs) • Necessary for priming adaptive immune responses 5 The immune system – a division of labor Adaptive immune system • Provides nearly unlimited diversity of receptors to protect the host from infection • B cells and T cells • Have unique receptors generated during development • B cells produce antibodies which help fight infection • T cells patrol for infected or cancerous cells • Recognize “foreign” or abnormal proteins on the cell surface • 100,000,000 unique T cells are present in all of us • Retains “memory” against infections and in some cases, cancer.