The Functions and Importance of Forests, with Applications to The
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

3. Water Quality
Table of Contents Table of Contents Table of Contents.................................................................................................................. i List of Tables ........................................................................................................................ v List of Figures....................................................................................................................... vii Acknowledgements............................................................................................................... xi Errata Sheet Issued May 4, 2011 .......................................................................................... xiii 1. Introduction........................................................................................................................ 1 1.1 What is the purpose and scope of this report? ......................................................... 1 1.2 What constitutes the New York City water supply system? ................................... 1 1.3 What are the objectives of water quality monitoring and how are the sampling programs organized? ........................................................................... 3 1.4 What types of monitoring networks are used to provide coverage of such a large watershed? .................................................................................................. 5 1.5 How do the different monitoring efforts complement each other? .......................... 9 1.6 How many water samples did DEP collect -

Comprehensive Plan Update North Salem Comprehensive Plan
NORTH SALEM COMPREHENSIVE PLAN UPDATE NORTH SALEM COMPREHENSIVE PLAN Prepared by: Town of North Salem Delancey Hall, Town Hall 266 Titicus Road North Salem, New York 10560 Adopted December 20, 2011 FERRANDINO & ASSOCIATES INC. DRAFT JAN NORTH SALEM COMPREHENSIVE PLAN Acknowledgments Town Board Warren Lucas, Supervisor Peter Kamenstein, Deputy Supervisor Stephen Bobolia, Councilman Mary Elizabeth Reeve, Councilwoman Amy Rosmarin, Councilwoman Comprehensive Planning Committee John White, Chair Janice Will, Secretary Martin Aronchick, Member Katherine Daniels, Member Linda Farina, Member Charlotte Harris, Member Robert Kotch, Member Michelle La Mothe, Member Drew Outhouse, Member Pam Pooley, Member Alan Towers, Member Peter Wiederhorn, Member Planning Consultant Ferrandino & Associates Inc. Planning and Development Consultants Three West Main Street, Suite 214 Elmsford, New York 10523 Vince Ferrandino, AICP, Principal-in-Charge Teresa Bergey, AICP, Senior Planner/Project Manager Kruti Bhatia, AICP, Planner Evan Smith, Planner In concert with Fitzgerald & Halliday Inc., for Transportation Mary Manning, P.E., Project Manager FERRANDINO & ASSOCIATES INC. DECEMBER 2011 NORTH SALEM COMPREHENSIVE PLAN Planning Board Cynthia Curtis, Chair Recreation Committee Dawn Onufrik, Secretary John Varachi, Chair Charlotte Harris, Member/ CPC Liaison Norma Bandak, Member Gary Jacobi, Member Andrew Brown, Member Bernard Sweeny, Member Brendan Curran, Member Robert Tompkins, Member Allison Hublard-Hershman, Member Della Mancuso, Member Zoning Board of Appeals -
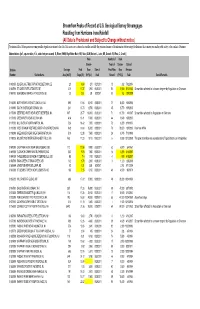
Streamflow Peaks of Record at US Geological Survey Streamgages
Streamflow Peaks of Record at U.S. Geological Survey Streamgages Resulting from Hurricane Irene Rainfall (All Data Is Provisional and Subject to Change without notice) Provisional data: Subsequent review may result in significant revisions to the data. Data users are cautioned to consider carefully the provisional nature of the information before using it for decisions that concern personal or public safety or the conduct of business Abbreviations: [mi2, square miles; ft3/s, cubic feet per second; R, River; HWM, High Water Mark; NR, Near; BLW, Below; L, Lake; BR, Branch; W, West; C, Creek ] Peak Number of Peak Stream‐ Years of Stream‐ Date of Station Drainage Peak flow Date of Peak Flow flow Previous Number Station Name Area (mi^2) Stage (ft) (ft^3/s) Peak Record (ft^3/s) Peak Special Remarks 01483155 SILVER LAKE TRIBUTARY AT MIDDLETOWN, DE 2.0 4.64 270 8/27/2011 10 212 7/12/2004 01483700 ST JONES RIVER AT DOVER, DE 31.9 11.72 1,900 8/28/2011 53 1,900 9/13/1960 Streamflow affected to unknown degree by Regulation or Diversion 01484100 BEAVERDAM BRANCH AT HOUSTON , DE 303.0 5575.57 300 8/28/2011 53 163 12/9/2009 01169000 NORTH RIVER AT SHATTUCKVILLE, MA 89.0 17.66 53,100 8/28/2011 71 18,800 10/9/2005 01169900 SOUTH RIVER NEAR CONWAY, MA 24.1 13.73 12,700 8/28/2011 45 8,770 10/9/2005 01170000 DEERFIELD RIVER NEAR WEST DEERFIELD, MA 557 23.77 103,000 8/28/2011 71 61,700 4/5/1987 Streamflow affected by Regulation or Diversion 01170100 GREEN RIVER NEAR COLRAIN, MA 41.4 13.31 17,500 8/28/2011 44 6,540 10/9/2005 01171500 MILL RIVER AT NORTHAMPTON, MA 52.6 16.42 7,000 8/28/2011 73 6,300 8/19/1955 01181000 WEST BRANCH WESTFIELD RIVER AT HUNTINGTON, MA 94.0 16.94 35,000 8/28/2011 76 28,000 10/9/2005 Peak from HWM. -

Westchester County Fishing Waters NYS Department of Health Fish Advisories & Publicly Accessible Waters
Westchester County Fishing Waters NYS Department of Health Fish Advisories & Publicly Accessible Waters Muscoot River N PUTNAM COUNTY Titicus Reservoir WESTCHESTER COUNTY Titcus Dam Titicus River Hollowbrook Dam Peekskill Hollow Brook Croton River Amawalk Reservoir Waccabuc Amawalk Dam River Blue Mohansic Muscoot River Mountain Cross River Lake Cross River Dam Reservation Reservoir Lounsbury Pond Dam New Croton Muscoot Cross River Dickey Brook Dam Reservoir Muscoot Reservoir New Croton Reservoir Dam Croton Water Supply Dams Croton RiverQuaker Bridge Dam Stone Hill River Silver Lake Dam All outlined waters are NYS DEC public access waters; there may be other fishing access sites in your county. General Advisory Applies Pocantico Lake Whole family: 4 fish meals/month Specific Advisory Applies Hudson River Swan Lake Women under 50 & children under 15: do not eat SwanDam Men over 15 & women over 50: health.ny.gov/fish/HV Lake Sleepy Hollow Dam Location Waterfall Dam Kensico Dam Stream Flow Connected Tributary, Advisory Applies Kensico Reservoir Woodlands Lake Woodlands Lake Dam Popham Road Dam Sheldrake River Long Island Sound* Saw Mill River *Some marine fish advisories exist Bronx River for women under 50 and children, Hodgman Dam visit www.health.ny.gov/fish/NYC for restrictions. The “Flume” www.health.ny.gov/fish [email protected] Map data © 2017 Google Bronx Zoo Double Dam 12/5/18. -

Series 252 NYC Watershed Proceedings
Westchester County Archives Series 252 2199 Saw Mill River Road Elmsford, New York 10523 NYC Watershed Proceedings – Volume Listing (914) 231-1500 1877‐1919 ITEM DATE CALL NUMBER Byram 1899‐1907 A‐0070 (1) Byram 1899‐1907 A‐0070 (2) Kensico Reservoir 1893‐1910 A‐0070 (3) Kensico Reservoir 1893‐1910 A‐0070 (4) Kensico Reservoir 1893‐1910 A‐0070 (5) Kensico Reservoir 1893‐1910 A‐0070 (6) Kensico Reservoir 1893‐1910 A‐0070 (7)S (J5) Rye Lake, Wampus River and Wampus Pond 1903 A‐0070 (8) Watershed Proceedings – Index for Kensico, n.d. A‐0070 (9) Byram, Rye Lake and Wampus Pond Mount Kisco – Sanitary Protection and 1893‐1901 A‐0070 (10) Lands to be Acquired Reservoir “M” 1896‐1897 A‐0070 (11)S (K3) Cornell Dam 1896‐1909 A‐0070 (12) Cornell Dam 1896‐1909 A‐0070 (13) Cornell Dam 1896‐1909 A‐0070 (14) Cornell Dam 1896‐1909 A‐0070 (15) Croton Aqueduct 1884‐1890 A‐0070 (16) Croton Aqueduct 1884‐1890 A‐0070 (17) Page 1 Westchester County Archives Series 252 2199 Saw Mill River Road Elmsford, New York 10523 NYC Watershed Proceedings – Volume Listing (914) 231-1500 1877‐1919 ITEM DATE CALL NUMBER Croton Aqueduct 1884‐1890 A‐0070 (18) Pipeline, Kensico and Rye Pond Reservoirs 1881‐1903 A‐0070 (19) (Liber 1) Pipeline, Kensico and Rye Pond Reservoirs 1881‐1903 A‐0070 (20) (Liber 2) Watershed Proceedings – Index for Cornell n.d. A‐0070 (21) Dam, Mount Kisco, New Aqueduct (Croton?), Pipeline, In Re Bronx “Reservoir A” in Somers – Sanitary 1877‐1900 A‐0070 (22) Protection Patterson and Brewster 1899‐1900 A‐0070 (23) Lake Kirk, Lake Mahopac and Muscoot River -

Series 252 NYC Watershed Proceedings – Alphabetical Listing
Westchester County Archives Series 252 2199 Saw Mill River Road Elmsford, New York 10523 NYC Watershed Proceedings – Alphabetical Listing (914) 231-1500 1877‐1919 ITEM DATE CALL NUMBER Brewster, Lake Gleneida, Farmers Mills, 1894‐1899 A‐0070 (26) White Pond Byram 1899‐1907 A‐0070 (1) Byram 1899‐1907 A‐0070 (2) Carmel, Lake Gleneida 1906 A‐0070 (27) Catskill Aqueduct 1907‐1910 A‐0070 (29) Cornell Dam 1896‐1909 A‐0070 (12) Cornell Dam 1896‐1909 A‐0070 (13) Cornell Dam 1896‐1909 A‐0070 (14) Cornell Dam 1896‐1909 A‐0070 (15) Cross River Dam and Reservoir 1809‐1910 A‐0070 (32) Cross River Dam and Reservoir 1809‐1910 A‐0070 (33) Croton Aqueduct 1884‐1890 A‐0070 (16) Croton Aqueduct 1884‐1890 A‐0070 (17) Croton Aqueduct 1884‐1890 A‐0070 (18) Croton Falls Dam and Reservoir 1906‐1910 A‐0070 (31) Farmers Mills, White Pond and Carmel 1896‐1904 A‐0070 (25) Hill View Reservoir 1908‐1917 A‐0070 (30) Westchester County Archives Series 252 2199 Saw Mill River Road Elmsford, New York 10523 NYC Watershed Proceedings – Alphabetical Listing (914) 231-1500 1877‐1919 ITEM DATE CALL NUMBER Jerome Park 1895‐1898 A‐0070 (28) Kensico Reservoir 1893‐1910 A‐0070 (3) Kensico Reservoir 1893‐1910 A‐0070 (4) Kensico Reservoir 1893‐1910 A‐0070 (5) Kensico Reservoir 1893‐1910 A‐0070 (6) Kensico Reservoir 1893‐1910 A‐0070 (7)S (J5) Lake Kirk, Lake Mahopac and Muscoot River 1879 A‐0070 (24) Mount Kisco – Sanitary Protection and 1893‐1901 A‐0070 (10) Lands to be acquired Patterson and Brewster 1899‐1900 A‐0070 (23) Pipeline, Kensico and Rye Pond Reservoirs 1881‐1903 A‐0070 -

Schenob Brook
Sages Ravine Brook Schenob BrookSchenob Brook Housatonic River Valley Brook Moore Brook Connecticut River North Canaan Watchaug Brook Scantic RiverScantic River Whiting River Doolittle Lake Brook Muddy Brook Quinebaug River Blackberry River Hartland East Branch Salmon Brook Somers Union Colebrook East Branch Salmon Brook Lebanon Brook Fivemile RiverRocky Brook Blackberry RiverBlackberry River English Neighborhood Brook Sandy BrookSandy Brook Muddy Brook Freshwater Brook Ellis Brook Spruce Swamp Creek Connecticut River Furnace Brook Freshwater Brook Furnace Brook Suffield Scantic RiverScantic River Roaring Brook Bigelow Brook Salisbury Housatonic River Scantic River Gulf Stream Bigelow Brook Norfolk East Branch Farmington RiverWest Branch Salmon Brook Enfield Stafford Muddy BrookMuddy Brook Factory Brook Hollenbeck River Abbey Brook Roaring Brook Woodstock Wangum Lake Brook Still River Granby Edson BrookEdson Brook Thompson Factory Brook Still River Stony Brook Stony Brook Stony Brook Crystal Lake Brook Wangum Lake Brook Middle RiverMiddle River Sucker BrookSalmon Creek Abbey Brook Salmon Creek Mad RiverMad River East Granby French RiverFrench River Hall Meadow Brook Willimantic River Barkhamsted Connecticut River Fenton River Mill Brook Salmon Creek West Branch Salmon Brook Connecticut River Still River Salmon BrookSalmon Brook Thompson Brook Still River Canaan Brown Brook Winchester Broad BrookBroad Brook Bigelow Brook Bungee Brook Little RiverLittle River Fivemile River West Branch Farmington River Windsor Locks Willimantic River First -

Town of Lewisboro Master Plan
Lewisboro Town Master Plan Town of Lewisboro Westchester County, New York Adopted by the Town of Lewisboro Planning Board May 24,'1985 Prepared by the Town of Lewisboro Planning Board Paul A. Lewis, Chairman John A. Armstrong Lee V. Blum Kenneth Batchelor Enzo V. Allegretti Marilyn J. Madsen, Secretary Theodore H. Chase (until December 1983) Donald H. Ed man (until November 1983) Carolyn U. Smith (until June 1983) William A. Best, Jr. (until August 1982) John J. Donovan (until March 1981 ) With the assistance of Frederick P. Clark Associates Planning Consultants, Rye, New York David J. Portman, Partner-in-Charge Edward E. Buroughs, Planner-in Charge Table of Contents Page INTRODUCTION vi LEWISBORO BASE MAP viii 1 . EXISTING LAND USE 1 1.1 Extent of Development 1 1.2 Characteristics of Land Use 1 1.3 Zoning 6 1.4 Development in Progress 7 2. POPULATION AND HOUSING 10 2. 1 Population Growth 10 2.2 Age Characteristics 12 2.3 Social Characteristics 14 2.4 Housing Growth 15 2.5 Housing Characteristics 17 2.6 Potential Residential Growth 18 3. PHYSICAL FEATURES OF THE LAND 22 3. 1 Topography and Surface Hydrology 22 3.2 Soils 24 3.3 Soils Characteristics 26 3.4 Wetland Functions 27 3. 5 Aquifers 29 3.6 Development Limitations Summary 31 4. THE REGIONAL CONTEXT 33 4.1 New York State 33 4.2 Tri-State Regional Planning Commission 34 4.3 Regional Plan Association 38 4.4 Westchester County 38 4.5 Adjacent Towns 44 5. FISCAL CONDITIONS 47 5.1 Sources of Revenue 47 5.2 Tax Base Trends 49 5. -

Relating Major Ions and Nutrients to Watershed Conditions Across a Mixed-Use, Water-Supply Watershed
J. N. Am. Benthol. Soc., 2006, 25(4):887–911 Ó 2006 by The North American Benthological Society Relating major ions and nutrients to watershed conditions across a mixed-use, water-supply watershed 1 2 3 Charles L. Dow , David B. Arscott , AND J. Denis Newbold Stroud Water Research Center, 970 Spencer Road, Avondale, Pennsylvania 19311 USA Abstract. Stream inorganic chemistry was sampled under summer baseflow conditions from 2000 to 2002 at 60 sites as part of a large-scale, enhanced water-quality monitoring project (the Project) across New York City’s drinking-water-supply watersheds. The 60 stream sites were evenly divided between regions east and west of the Hudson River (EOH and WOH, respectively). EOH sites had generally higher ionic concentrations than WOH sites, reflecting differences in land use and geology. Within each region, variability in inorganic chemistry data between sites was far greater than annual variability within sites. Geology was an important factor controlling underlying baseflow chemistry differences within and between regions. However, after taking into account geological controls, anthropogenic land uses primarily defined ion and nutrient baseflow chemistry patterns at regional and watershed levels. In general, watershed-scale landscape attributes had either the strongest relationships with analytes or had relationships with analytes that did not differ fundamentally from relationships of riparian- or reach- scale landscape attributes. Individual analyses indicated no dominant watershed-scale landscape attribute that could be used to predict instream inorganic chemistry concentrations, and no single ion or nutrient was identified as the best indicator of a given anthropogenic land use. Our results provide a comprehensive baseline of information for future water-quality assessments in the region and will aid in examining other components of the Project. -

Flood of January 19-20, 1996 in New York State
Flood of January 19-20, 1996 in New York State By Richard Lumia U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 97-4252 Prepared in cooperation with the IISRS New York State Department of Transportation science for a changing world Albany, New York 1998 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Thomas J. Casadevall, Acting Director The use of trade, product, or firm names in this report is for identification or location purposes only and does not constitute endorsement of products by the U.S. Geological Survey, nor impute responsibility for any present or potential effects on the natural resources of the United States. For addtional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services 425 Jordan Road Box 25286 Troy, New York 12180 Denver, CO 80225-0286 CONTENTS Page Abstract....................................................................................... 1 Introduction.................................................................................... 1 Purpose and Scope ......................................................................... 2 Acknowledgments ......................................................................... 3 Physiography and Climate of New York and the Catskill Mountain Region .................................. 4 Physiography.............................................................................. 4 Climate ................................................................................. -

Contents (294
vi CONTENTS Page New York district office locations and addresses . iii Preface. iv List of surface-water stations, in downstream order, for which records are published. vii List of ground-water wells, by county, for which records are published . ix List of discontinued surface-water discharge or stage-only stations . x List of discontinued surface-water-quality stations . xiv List of discontinued crest-stage partial-record stations . xv Introduction. 1 Cooperation. 1 Summary of hydrologic conditions. 2 Surface Water . 2 Water Quality. 8 Ground Water . 8 Special networks and programs . 10 Explanation of records . 10 Station identification numbers . 10 Downstream order system. 11 Latitude-longitude system. 11 Records of stage and water discharge. 11 Data collection and computation . 11 Data presentation . 12 Station manuscript . 12 Data table of daily mean values . 13 Statistics of monthly mean data . 13 Summary statistics . 13 Hydrographs . 14 Identifying estimated daily discharge . 14 Accuracy of the records . 14 Other records available . 15 Records of surface-water quality . 15 Classification of records . 15 Arrangement of records . 15 On-site measurements and sample collection. 15 Water temperature. 16 Sediment . 16 Laboratory measurements. 16 Data presentation . 16 Categories of water-quality data . 17 Frequency-of-sampling notation. 17 Dissolved trace-element concentrations . 17 Change in National Trends Network procedures . 17 Remarks codes . 18 Quality-control data . .. -

Water Quality Monitoring in the Source Water Areas for New York City: In
Water Quality Monitoring in the Source Water Areas for New York City: An Integrative Watershed Approach A Report on Year 5 (2004) Monitoring Activities Submitted by: Stroud Water Research Center Contribution No. 2005007 970 Spencer Road Avondale, PA 19311 27 October 2005 NY WATERSHEDS PROJECT – YEAR 5 REPORT – 27 OCTOBER 2005 Report Prepared by: David B. Arscott, Ph.D. Anthony K. Aufdenkampe, Ph.D. Thomas L. Bott, Ph.D. Charles L. Dow, Ph.D. John K. Jackson, Ph.D. Louis A. Kaplan, Ph.D. J. Denis Newbold, Ph.D. Bernard W. Sweeney, Ph.D. - II - NY WATERSHEDS PROJECT – YEAR 5 REPORT – 27 OCTOBER 2005 Table of Contents Chapter 1 - Introduction..............................................................................................................- 1 - Literature cited.....................................................................................................................................................- 3 - Chapter 2 - Technical Design .....................................................................................................- 5 - Overview..............................................................................................................................................................- 5 - Phase II Study Site Descriptions..........................................................................................................................- 7 - Literature Cited..................................................................................................................................................-