CPT/HCPCS Medication Code Reference List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Xenon-Xenon Collisions in the Lhc
9th International Particle Accelerator Conference IPAC2018, Vancouver, BC, Canada JACoW Publishing ISBN: 978-3-95450-184-7 doi:10.18429/JACoW-IPAC2018-MOPMF039 FIRST XENON-XENON COLLISIONS IN THE LHC M. Schaumann∗, R. Alemany-Fernandez, P. Baudrenghien, T. Bohl, C. Bracco, R. Bruce, N. Fuster-Martinez, M. A. Jebramcik, J.M. Jowett, T. Mertens, D. Mirarchi, S. Redaelli, B. Salvachua, M. Solfaroli, H. Timko, J. Wenninger, CERN, Geneva, Switzerland Abstract EXECUTION OF THE RUN In 2017, the CERN accelerator complex once again demonstrated its flexibility by producing beams of a new A total of about 18 h of LHC time were taken for the Xe ion species, xenon, that were successfully injected into LHC. collision run. The schedule was designed to include set-up, On 12 October, collisions of fully stripped xenon nuclei first injection, validation and physics data taking. A further 12 h were devoted to experiments on crystal collimation, de- were recorded for the first time in the LHC√ at a centre-of- scribed elsewhere [3]. This tight schedule was only feasible mass energy per colliding nucleon pair of sNN = 5.44 TeV. Physics data taking started 9.5hafter the first injection of by drastically reducing the complexity of the set-up follow- xenon beams and lasted a total of 6h. The integrated lumi- ing the model of the p–Pb pilot run in September 2012 [4,5] nosity delivered to the four LHC experiments was sufficient when first injection, validation and physics data-taking were that new physics results can be expected soon. We provide achieved within a single fill. -

XENON X-1100 High-Intensity Pulsed Light System
XENON X-1100 High-Intensity Pulsed Light System The low cost R&D tool for investigating the applicability of Pulsed Light for new and emerging applications. The tool that researchers, scientists and engineers have been looking for is here. An easy-to-use photonic system developed by XENON that will open new doors and lead to new discoveries. The power of Pulsed Light In virtually all disciplines of science and technology there are applications where precise delivery of energy is demanded. One less studied energy delivery mechanism is the use of high-intensity pulsed light. At present the most prevalent example of this is in the use of lasers. However, the point source, coherent and single wavelength nature of lasers, are often not suited to the majority of challenges facing many industries. In these situations, it is crucial to have a broad spectra source so as not to be restricted in choosing materials with specific absorption characteristics. Because XENON sources generate light which extends from the deep UV to IR and often with peak pulse powers measured in megawatts, the ability of these sources to perform tasks such as breaking chemical bonds, sintering, ablating or sterilizing is highly realizable. The high peak powers generated by XENON’s production level systems are possible by tightly controlling the pulse durations measured from a few microseconds to milliseconds. Until now there was no practical method of generating this intense pulsed light in a low-cost R&D tool that was safe, compact and versatile. XENON has developed a system to address this challenge based on over 50 years of exper- tise in the Pulsed Light industry. -

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -

Pharmacotherapy of Impaired Mucociliary Clearance in Non-CF Pediatric Lung Disease
Pediatric Pulmonology 42:989–1001 (2007) State of the Art Pharmacotherapy of Impaired Mucociliary Clearance in Non-CF Pediatric Lung Disease. A Review of the Literature 1 1 1,2 Ruben Boogaard, MD, * Johan C. de Jongste, MD, PhD, and Peter J.F.M. Merkus, MD, PhD Summary. Mucoactive agents are used to treat a variety of lung diseases involving impaired mucociliary clearance or mucus hypersecretion. The mucoactive agents studied most frequently are N-acetylcysteine (NAC), recombinant human DNase (rhDNase), and hypertonic saline. Studies on the efficacy of these have been mainly conducted in adults, and in patients with cystic fibrosis (CF). The exact role of mucoactive agents in children with non-CF lung disease is not well established. We present an overview of the current literature reporting clinical outcome measures of treatment with NAC, rhDNase, and hypertonic saline in children. Pediatr Pulmonol. 2007; 42:989–1001. ß 2007 Wiley-Liss, Inc. Key words: mucolytic; sulfhydryl compounds; N-acetylcysteine; dornase alfa; hyper- tonic saline; respiratory tract disease. INTRODUCTION One possible means to evaluate a mucoactive agent is to assess its effect on mucociliary clearance (MCC) or cough Mucus clearance is an important primary innate airway clearance with the use of radiolabeled aerosol. Discussing defense mechanism, and our understanding of the key this subject is outside the scope of this review. Moreover, parameters underlying its function has grown rapidly in the studies on mucoactive agents in CF patients, and studies last decade.1,2 Impaired mucus clearance or mucus hyper- on physiotherapy or secretion clearance techniques in secretion are important clinical features in diseases such as (pediatric) lung disease patients have been reviewed by cystic fibrosis (CF), recurrent bronchitis, asthma, and others, and will therefore not be discussed in this review. -

Supportive Care Medications
th Clinical Pharmacy Guide: Cancer Drug Treatment Assessment and Review 5 Edition Supportive Care Medications Contents Introduction ................................................................................................................. 2 Supportive Care Information in Protocols .................................................................... 2 Antidiarrheals .............................................................................................................. 5 Loperamide ............................................................................................................. 5 Antiemetics .................................................................................................................. 6 Emetogenicity .......................................................................................................... 7 Types of Chemotherapy-Induced Nausea and Vomiting .......................................... 7 Classifications of Antiemetics .................................................................................. 8 Anti-infective Agents .................................................................................................. 10 Antibiotics .............................................................................................................. 10 Antivirals ................................................................................................................ 11 Arthralgias/Myalgias ................................................................................................. -

Xenon Gas Xe Safety Data Sheet SDS P4677
Xenon Safety Data Sheet P-4677 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Date of issue: 01/01/1979 Revision date: 10/24/2016 Supersedes: 10/02/2014 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Substance Name : Xenon CAS No : 7440-63-3 Formula : Xe Other means of identification : Xenon 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Industrial use. Use as directed. 1.3. Details of the supplier of the safety data sheet Praxair, Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA T 1-800-772-9247 (1-800-PRAXAIR) - F 1-716-879-2146 www.praxair.com 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS-US classification Compressed gas H280 2.2. Label elements GHS-US labeling Hazard pictograms (GHS-US) : GHS04 Signal word (GHS-US) : WARNING Hazard statements (GHS-US) : H280 - CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED OSHA-H01 - MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION Precautionary statements (GHS-US) : P202 - Do not handle until all safety precautions have been read and understood P271+P403 - Use and store only outdoors or in a well-ventilated place CGA-PG05 - Use a back flow preventive device in the piping CGA-PG10 - Use only with equipment rated for cylinder pressure CGA-PG06 - Close valve after each use and when empty CGA-PG02 - Protect from sunlight when ambient temperature exceeds 52°C (125°F) 2.3. -

Lung Ventilation - Xenon
Lung Ventilation - Xenon Special Instructions Patients with severe dyspnea may not be able to tolerate this examination. To be performed only at UNMH. Radiopharmaceutical: Xe-133 gas Dose (Adult/Pediatric): Refer to Nuclear Medicine Dose Chart Route of Administration: Inhalation from a Xe-133 gas delivery system. Patient Preparation: None. Equipment Setup: Gamma Camera: ZOOM as appropriate for small children Collimator: Orbiter: LEAP SPECT-CT/ECAM/Evo/Symbia E: High resolution Computer setup: • Static acquisition • 128 x 128 matrix • Zoom 1.0 (greater for small children)—ensure that the lungs fill a significant portion of the detector for small patients • 10 sec/image for inspiration • 30 sec/image for all other images Patient Positioning: Orbiter: • Seated upright on a stool (preferred) with the detector posterior to the patient. Supine if necessary All others: • Supine Procedure: • If the patient is able to sit upright on a stool for the examination, perform the ventilation portion of the examination on the Orbiter if available. • Before beginning the study, explain the breathing maneuvers required; the patient should rehearse these maneuvers. • Place the mask on the patient and ensure that it fits snugly. • Inspiration (Single-breath) Image: • Instruct the patient to exhale completely and then to take a deep breath as the Xe-133 gas flows in. • The patient should hold his/her breath for 10 sec (if possible) while posterior (and anterior if on a dual-headed camera) imaging is performed. Revised 2017-01 Lung Ventilation - Xenon (continued) • Equilibrium (Rebreathing/Wash-in) Images: • The patient should then breathe normally for 3 images (90 seconds). -

Table III: 2019 Medicare Drug Fee Schedule* CY 2019 4Th Quarter
Table III: 2019 Medicare Drug Fee Schedule* CY 2019 4th Quarter Average Sales Price (ASP) Data Plus 6 Percent *The Medicare payments allowance limits are effective Oct. 1 through Dec. 31, 2019. CY 2019 CY 2019 CY 2019 CY 2019 Effective Jan. 1 Effective Apr. 1 Effective July 1 Effective Oct. 1 Through Mar. 31 Through June 30 Throught Sept. 30 Through Dec. 31 Code Description Code Dosage ASP plus 6 percent ASP Plus 6 percent ASP Plus 6 percent ASP plus 6 percent J0129 Abatacept 10mg $51.61 $52.49 $53.94 $54.32 J0130 Abciximab injection 10mg $1,429.07 $1,429.07 $1,429.07 $1,429.07 J0133 Acyclovir, 5 mg 5mg $0.05 $0.05 $0.04 $0.04 J0171 Adrenalin epinephrin inject 0.1mg $0.74 $0.76 $0.86 $0.83 J0207 Amifostine 500mg $979.75 $990.39 $977.31 $1,003.59 J0256 Alpha 1 proteinase inhibitor 10mg $4.55 $4.56 $4.51 $4.55 J0280 Aminophyllin 250 MG inj 250mg $7.02 $7.33 $7.33 $10.88 J0289 Amphotericin b liposome inj 10mg $22.19 $23.92 $18.32 $26.09 J0295 Ampicillin sodium per 1.5 gm 1.5gm $2.62 $2.81 $3.32 $2.06 J0360 Hydralazine hcl injection 20mg $2.58 $2.58 $4.07 $4.21 J0456 Azithromycin 500mg $2.76 $2.58 $2.75 $3.05 J0461 Atropine sulfate injection 0.01mg $0.07 $0.07 $0.07 $0.06 J0475 Baclofen 10 MG injection 10mg $169.75 $174.19 $173.39 $169.51 J0476 Baclofen intrathecal trial 50mcg $44.35 $49.98 $50.44 $50.05 J0480 Basiliximab 20mg $3,677.61 $3,687.17 $3,789.63 $3,799.93 J0490 Belimumab 10mg $44.16 $44.16 $44.82 $44.82 J0500 Dicyclomine injection 20mg $69.65 $82.33 $69.97 $57.58 J0515 Inj benztropine mesylate 1mg $18.67 $18.75 $19.89 $17.74 J0570 -

Mucoactive Agents for Airway Mucus Hypersecretory Diseases
Mucoactive Agents for Airway Mucus Hypersecretory Diseases Duncan F Rogers PhD FIBiol Introduction Sputum Profile of Airway Inflammation and Mucus Hypersecretory Phenotype in Asthma, COPD, and CF Which Aspect of Airway Mucus Hypersecretion to Target? Theoretical Requirements for Effective Therapy of Airway Mucus Hypersecretion Current Recommendations for Clinical Use of Mucolytic Drugs Mucoactive Drugs N-Acetylcysteine: How Does it Work? Does it Work? Dornase Alfa Hypertonic Saline Surfactant Analysis Summary Airway mucus hypersecretion is a feature of a number of severe respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF). However, each disease has a different airway inflammatory response, with consequent, and presumably linked, mucus hypersecretory phenotype. Thus, it is possible that optimal treatment of the mucus hyper- secretory element of each disease should be disease-specific. Nevertheless, mucoactive drugs are a longstanding and popular therapeutic option, and numerous compounds (eg, N-acetylcysteine, erdosteine, and ambroxol) are available for clinical use worldwide. However, rational recommen- dation of these drugs in guidelines for management of asthma, COPD, or CF has been hampered by lack of information from well-designed clinical trials. In addition, the mechanism of action of most of these drugs is unknown. Consequently, although it is possible to categorize them according to putative mechanisms of action, as expectorants (aid and/or induce cough), mucolytics (thin -
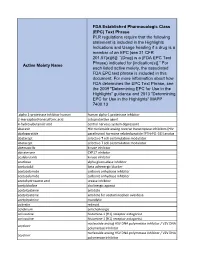
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Pharmacokinetics and Pharmacology of Drugs Used in Children
Drug and Fluid Th erapy SECTION II Pharmacokinetics and Pharmacology of Drugs Used CHAPTER 6 in Children Charles J. Coté, Jerrold Lerman, Robert M. Ward, Ralph A. Lugo, and Nishan Goudsouzian Drug Distribution Propofol Protein Binding Ketamine Body Composition Etomidate Metabolism and Excretion Muscle Relaxants Hepatic Blood Flow Succinylcholine Renal Excretion Intermediate-Acting Nondepolarizing Relaxants Pharmacokinetic Principles and Calculations Atracurium First-Order Kinetics Cisatracurium Half-Life Vecuronium First-Order Single-Compartment Kinetics Rocuronium First-Order Multiple-Compartment Kinetics Clinical Implications When Using Short- and Zero-Order Kinetics Intermediate-Acting Relaxants Apparent Volume of Distribution Long-Acting Nondepolarizing Relaxants Repetitive Dosing and Drug Accumulation Pancuronium Steady State Antagonism of Muscle Relaxants Loading Dose General Principles Central Nervous System Effects Suggamadex The Drug Approval Process, the Package Insert, and Relaxants in Special Situations Drug Labeling Opioids Inhalation Anesthetic Agents Morphine Physicochemical Properties Meperidine Pharmacokinetics of Inhaled Anesthetics Hydromorphone Pharmacodynamics of Inhaled Anesthetics Oxycodone Clinical Effects Methadone Nitrous Oxide Fentanyl Environmental Impact Alfentanil Oxygen Sufentanil Intravenous Anesthetic Agents Remifentanil Barbiturates Butorphanol and Nalbuphine 89 A Practice of Anesthesia for Infants and Children Codeine Antiemetics Tramadol Metoclopramide Nonsteroidal Anti-infl ammatory Agents 5-Hydroxytryptamine -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole