Dr. Janet Woodcock
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Janet Woodcock, MD Director of the Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA)
Janet Woodcock, MD Director of the Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA) Janet Woodcock is the director of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA). The center makes sure that safe and effective drugs are available to improve the health of people in the United States. Dr. Woodcock and her center: • evaluate prescription and over the counter drugs before they can be sold and oversee their testing in clinical trials • provide health care professionals and patients the information they need to use medicines wisely • ensure that drugs, both brand-name and generic, work correctly and that their health benefits outweigh their known risks • take action against unapproved, contaminated, or fraudulent drugs that are marketed illegally "New drugs—and new uses for older drugs—save lives, reduce suffering, and improve the quality of life for millions of Americans," says Dr. Woodcock. "I am continually challenged to make sure that FDA’s regulatory process remains the world's gold standard for drug approval and safety." Dr. Woodcock has led many of FDA's drug initiatives. She introduced the concept of risk management in 2000 as a new approach to drug safety. Since 2002, she has led the "Pharmaceutical Quality for the 21st Century Initiative," FDA's highly successful effort to modernize drug manufacturing and its regulation. In 2004, she introduced FDA's "Critical Path" Initiative, which is designed to move medical discoveries from the laboratory to consumers more efficiently. Most recently, Dr. Woodcock launched the "Safety First" and "Safe Use" initiatives designed to improve drug safety management within and outside FDA, respectively. -

Welcome to the 2021 Stanford Drug Discovery Symposium
WELCOME & REGISTRATION Welcome to the 2021 Stanford Drug Discovery Symposium The Stanford Drug Discovery Symposium is an exciting forum to hear about drug discovery from leaders in the field. Speakers include leaders at major pharmaceutical companies, biotech industry, federal policy makers, venture capital firms, editors from major publications, and scientists making groundbreaking advances. The meeting also provides perspectives into how COVID-19 has led to change in these landscapes. We look forward to your participation in this exciting event. REGISTRATION https://tinyurl.com/SDDS2021 DAY-OF VIEWING PAGE https://tinyurl.com/sdds2021-livestream SOCIAL MEDIA #SDDS2021 Participant Interaction Platform SDDS will be using Slido as a platform to send questions to the speakers. Please note that the speakers may not have time to answer all questions during the panel sessions. Slido is accessible using the following formats: Webpage: Simply type your question in the Slido interface to the right of the video box on the viewing webpage. New browser window: Open the Slido website in a new browser window and enter participant code #SDDS2021 in the "Joining as a Participant" field at the top of the page. Cellphone: You can use Slido on your mobile phone (no app download required). Just scan the QR code to the right. SCHEDULE - MONDAY, APRIL 19 Time (PDT) WELCOME TO DAY 1 9:00 am Joseph Wu, MD, PhD Director, Stanford Cardiovascular Institute 9:05 am Marc Tessier-Lavigne, PhD President, Stanford University 9:10 am Lloyd Minor, MD Dean, Stanford School of Medicine SESSION I: BIOLOGY OF DISEASE Moderators: Helen Blau, PhD, and Mark Mercola, PhD 9:15 am Brian Kobilka, MD Helene Irwin Fagan Chair in Cardiology, Stanford University; Nobel prize in 2012 Challenges in Drug Discovery for G Protein Coupled Receptors 9:35 am Roger Kornberg, PhD Mrs. -

Welcome to FDA's Rare Disease Day 2021 Virtual Event
Welcome to FDA’s Rare Disease Day 2021 Virtual Event Webcast link: https://fda.yorkcast.com/webcast/Play/84f0dfdc2c1444679516d9f73d690b351d In this packet you will find: - Agenda - Conference Speaker and Moderator Brief Biographies 1 Agenda for FDA Rare Disease Day 2021: Friday, March 5, 2021 (virtual) 9:00-9:10am Welcome Janet Maynard, MD, MHS, Director, Office of Orphan Products Development (OOPD) FDA 9:10-9:15am Meeting Overview Lewis Fermaglich, MD, MHA, Acting Senior Clinical Advisor, OOPD, FDA 9:15-9:30am Orphan Products Grants Program Katherine Needleman, PhD, Director, Clinical Trials and Natural History Grant Program, OOPD, FDA 9:30-9:40am Opening Remarks Amy Abernethy, MD, PhD, Principal Deputy Commissioner of Food and Drugs, Acting Chief Information Officer, FDA 9:40-10:35am Panel 1: Rare Disease Partnerships, Collaborations, and Scientific Advancements Session Goal/Overview: Provide perspectives on successful partnerships to support rare disease product development. Outline the importance of working with rare disease stakeholders to ensure scientific advancements support the development of rare disease products. Mr. Kroslowitz and Dr. McCormack will provide presentations at the start of the panel followed by a facilitated discussion with all panel members. Moderator: Susan McCune, MD, Director, Office of Pediatric Therapeutics, FDA Panelists: • Robert Kroslowitz, President and CEO of Berlin Heart Inc. • Frank McCormack, MD, Professor of Medicine; Director, Division of Pulmonary, Critical Care, and Sleep Medicine, University -

The Administrative Law Review Is ACCEPTING SUBMISSIONS for Recent Developments
ADMINISTRATIVE LAW REVIEW Volume 63 Summer 2011 Number 3 REPORT Lobbying Law in the Spotlight: Challenges and Proposed Improvements ................................................... 419 ARTICLES An Inductive Understanding of Separation of Powers ............................................................. Jack M. Beermann 467 An Empirical Study of Judicial Review of Agency Interpretations of Agency Rules ........................ Richard J. Pierce, Jr. 515 Joshua Weiss Rule by Reasonableness ................................................... David Zaring 525 COMMENTS The Department of Veterans Affairs’ Entitlement Complex: Attorney Fees and Administrative Offset After Astrue v. Ratliff .............................................. Stacy L.Z. Edwards 561 The Illusion of Interchangeability: The Benefits and Dangers of Guidance-Plus Rulemaking in the FDA’s Biosimilar Approval Process ............... Jonathan Stroud 599 RECENT DEVELOPMENT Fees From Mars: Why the FTC Needs to Regulate Mortgage Assistance Relief Services (MARS) Fees .................. Alexander Lutch 645 ADMINISTRATIVE LAW REVIEW Volume 63 Summer 2011 Number 3 Editor in Chief Stacy L.Z. Edwards Executive Editor Managing Editor Keeley McCarty Brittany Ericksen Senior Recent Senior Senior Note & Senior Developments Editor Articles Editors Comment Editor Symposia Editor Emily Baver Aaron M. Moore Shannon L. Cain Jonathan Stroud Tom Rath Articles Editors Note & Comment Editors Sarah Fech Christina Andreen Carolyn Lindley David Van Fleet Bloys Amanda Lutz Aaron V. Gleaton Gregory M. Reyes Ted Uliassi Michael T. Vasquez Katherine A. Weatherford Topics and Trends Managers Jessica Buonaccorsi Kimberly Payne Senior Staff Katherine Aljinovic Brenda A. González Sapna Mehta Patrick Schultz Alexander Bard Elizabeth F. Jackson Diana M. Pak Yi Elizabeth S. Shen Kristan Callahan Harrison Kang Amanda K. Patton Ann Slacter Lauren Caplan Maanasa Kona Chris Pepe Sarah V. Stanley Robert D. Cardina Erin Kuhls Ricardo L. Piereck Dennis Tristani Sarah Chiang Priya Lamba Jennifer Ponder Sean S. -

August 8, 2018 Guidance Document Submission Dr. Janet Woodcock Dr
August 8, 2018 Guidance Document Submission Dr. Janet Woodcock Dr. Richard Pazdur Center for Drug Evaluation and Research Oncology Center of Excellence Food and Drug Administration Food and Drug Administration 10903 New Hampshire Ave 10903 New Hampshire Ave Silver Spring, MD 20993-0002 Silver Spring, MD 20993-0002 Dear Drs. Janet Woodcock and Richard Pazdur, and colleagues at the FDA, The American Society of Clinical Oncology (ASCO) and Friends of Cancer Research (Friends) formally submit the following draft guidance documents for consideration by the Food and Drug Administration (FDA). The content and strategies to modernize eligibility criteria for oncology clinical trials build upon recommendations developed by a consortium of stakeholders composed of patient advocates, drug/biotech manufacturers, investigators, and regulators. In 2016, ASCO and Friends began a joint project to develop and advance specific strategies to change the exclusionary nature of cancer clinical trial eligibility criteria on the following topics: 1) Brain Metastases, 2) HIV/AIDS, 3) Organ Dysfunction, 4) Prior and Concurrent Malignancies, and 5) Minimum Age for Enrollment. An ASCO-Friends joint research statement and four supporting manuscripts containing consensus recommendations based on the review of evidence, consideration of the patient population, and consultation with the research community were published in the Journal of Clinical Oncology on October 2, 2017. Since the publication of these recommendations, ASCO and Friends have been working to advance their broad implementation. To further bolster that effort, recommendations outlined in the published manuscripts have been adapted to serve as the foundation for the five FDA draft guidance documents enclosed in this submission. The recommendations aim to maximize the generalizability of clinical trial results while also maintaining the safety of clinical trial participants. -

Review of the Proposed Generic Drug and Biosimilars User Fees and Further Exam- Ination of Drug Shortages
REVIEW OF THE PROPOSED GENERIC DRUG AND BIOSIMILARS USER FEES AND FURTHER EXAM- INATION OF DRUG SHORTAGES HEARING BEFORE THE SUBCOMMITTEE ON HEALTH OF THE COMMITTEE ON ENERGY AND COMMERCE HOUSE OF REPRESENTATIVES ONE HUNDRED TWELFTH CONGRESS SECOND SESSION FEBRUARY 9, 2012 Serial No. 112–114 ( Printed for the use of the Committee on Energy and Commerce energycommerce.house.gov U.S. GOVERNMENT PRINTING OFFICE 81–517 PDF WASHINGTON : 2013 For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate Aug 31 2005 15:22 Jul 01, 2013 Jkt 037690 PO 00000 Frm 00001 Fmt 5011 Sfmt 5011 F:\112-11~1\112-11~1 WAYNE COMMITTEE ON ENERGY AND COMMERCE FRED UPTON, Michigan Chairman JOE BARTON, Texas HENRY A. WAXMAN, California Chairman Emeritus Ranking Member CLIFF STEARNS, Florida JOHN D. DINGELL, Michigan ED WHITFIELD, Kentucky Chairman Emeritus JOHN SHIMKUS, Illinois EDWARD J. MARKEY, Massachusetts JOSEPH R. PITTS, Pennsylvania EDOLPHUS TOWNS, New York MARY BONO MACK, California FRANK PALLONE, JR., New Jersey GREG WALDEN, Oregon BOBBY L. RUSH, Illinois LEE TERRY, Nebraska ANNA G. ESHOO, California MIKE ROGERS, Michigan ELIOT L. ENGEL, New York SUE WILKINS MYRICK, North Carolina GENE GREEN, Texas Vice Chairman DIANA DEGETTE, Colorado JOHN SULLIVAN, Oklahoma LOIS CAPPS, California TIM MURPHY, Pennsylvania MICHAEL F. DOYLE, Pennsylvania MICHAEL C. BURGESS, Texas JANICE D. SCHAKOWSKY, Illinois MARSHA BLACKBURN, Tennessee CHARLES A. GONZALEZ, Texas BRIAN P. BILBRAY, California JAY INSLEE, Washington CHARLES F. -

Food and Drug Law Journal
FOOD AND DRUG LAW JOURNAL EDITOR IN CHIEF Judy Rein EDITORIAL ADVISORY BOARD CHAIR VICE CHAIR FACULTY ADVISOR Laurie Lenkel Robert Giddings Joseph A. Page FDA – OC Hutchison PLLC Georgetown University Law Center _______________________________ Anthony Anscombe Kimberly Gold Sandra Retzky Sedgwick LLP Norton Rose Fulbright FDA – CTP LLP Peter Barton Hutt Jessica Ringel Covington & Burling John Johnson King & Spalding LLP FDA Imports Barbara Binzak Joan Rothenberg Blumenfeld Alan Katz FDA - CFSAN Buchanan Ingersoll & toXcel, LLC Jodi Schipper Rooney PC Sara Koblitz FDA – CDER Kellie Combs Hyman, Phelps & Christopher van Gundy Ropes & Gray LLP McNamara, PC Keller and Heckman Nathan Cortez Valerie Madamba Southern Methodist Blue Apron James Woodlee University Kleinfeld Kaplan & Becker LLP Alan Minsk Brian Dahl Arnall Golden Gregory Emily Wright Dahl Compliance LLP Pfizer Consulting LLC Nicole Negowetti Kimberly Yocum Sandra dePaulis Harvard Law School TC Heartland LLC FDA – CVM James O’Reilly Lowell Zeta University of Ian Fearon Cincinnati Hogan Lovells British American Tobacco Francis Palumbo Patricia Zettler Abraham Gitterman University of Maryland Georgia State Arnold & Porter LLP School of Pharmacy University Law School OFFICERS OF THE FOOD AND DRUG LAW INSTITUTE CHAIR: Jeffrey N. Gibbs, Hyman, Phelps & McNamara, P.C. TREASURER: Frederick R. Ball, Duane Morris LLP GENERAL COUNSEL/SECRETARY: Joy J. Liu, Vertex Pharmaceuticals IMMEDIATE PAST CHAIR: Allison M. Zieve, Public Citizen Litigation Group PRESIDENT & CEO: Amy Comstock Rick GEORGETOWN UNIVERSITY LAW CENTER GEORGETOWN LAW STUDENT EDITORS EDITOR IN CHIEF Erik Rynko MANAGING EDITORS Meaghan Jerrett Tiffany Weston ARTICLES & NOTES EDITOR SYMPOSIUM EDITOR Allison Parr Laura Higbee EXECUTIVE EDITORS Natalie Camastra Nicholas Hill Thomas Sanford Lacey Henry Daniel Krisch SENIOR STAFF EDITORS Seth Appiah-Opoku Daniel Elkus Nicholas Prust Emma Chapman Adam Harris GEORGETOWN LAW FACULTY FACULTY ADVISOR Joseph A. -

(PREPP) Initiative: Summary Report January 2021 PREPP Initiative: Summary Report January 2021
FDA COVID-19 Pandemic Recovery and Preparedness Plan (PREPP) Initiative: Summary Report January 2021 PREPP Initiative: Summary Report January 2021 Foreword The COVID-19 pandemic represents the most profound disruption to daily life in generations. It has reshaped our lives, forcing us to adapt the ways in which we live and work, producing enormous economic hardships, and causing profound personal pain and tragedy – with more than 350,000 deaths and 20 million confirmed cases in the United States alone – and contributed to enormous economic dislocation all over the world. The U.S. Food and Drug Administration (FDA or the “Agency”) has been at the forefront of the nation’s response to the pandemic. Our resourceful and resilient workforce of nearly 18,000 strong has continued to make unparalleled contributions and demonstrated unwavering commitment to promote and protect the health and safety of the American public. As the pandemic evolves, there are beacons of hope that we must not lose sight of, including the FDA’s emergency use authorization of COVID-19 vaccines, monoclonal antibody therapies, and rapid, more readily accessible tests for COVID-19, such as over-the-counter diagnostic tests. The pandemic has presented new challenges for the entire health care community and all levels of government, including the FDA. To carry out our mission during the pandemic, we continue to make decisions guided by science and the best evidence, even when the complete safety and efficacy information of a given product may not yet be known or available at the time. We will revise our policies as our understanding of the science changes based on the rapidly evolving data on this previously unknown, highly contagious virus. -
January 27, 2021 the Honorable Xavier Becerra Secretary of Health and Human Services-Designate U.S
January 27, 2021 The Honorable Xavier Becerra Secretary of Health and Human Services-Designate U.S. Department of Health and Human Services 200 Independence Avenue, S.W. Washington, D.C. 20201 The Honorable Norris Cochran Acting Secretary of Health and Human Services U.S. Department of Health and Human Services 200 Independence Avenue, S.W. Washington, D.C. 20201 Dear Secretary-Designate Becerra and Acting Secretary Cochran: We, the undersigned organizations, are writing to communicate our strong opposition to Dr. Janet Woodcock’s potential nomination for Commissioner of the U.S. Food and Drug Administration (FDA). We strongly urge that her position as Acting Commissioner be a very short transition prior to President Joe Biden nominating a Commissioner who has a track record focused on public health. Serving as the Director of the FDA’s Center for Drug Evaluation and Research (CDER) for more than 25 years, Dr. Woodcock presided over one of the worst regulatory agency failures in U.S. history. Since 1999, more than 500,000 Americans have lost their lives to opioid-related overdoses, and millions of Americans have developed opioid use disorder from use of prescription opioids. As a federal judge presiding over hundreds of county and state cases against opioid manufacturers and distributors recently wrote: “It is accurate to describe the opioid epidemic as a man-made plague, 20 years in the making.”1 Much of the responsibility for the opioid crisis clearly rests with industry. But the fact that opioid manufacturers for decades disseminated false claims about the risks and benefits of opioids points to a dereliction of duty by CDER, the FDA division led by Dr. -

Priorities for Personalized Medicine
PRESIDENT’S COUNCIL OF ADVISORS ON SCIENCE AND TECHNOLOGY • SEPTEMBER 2008 ★ ★ ★ ★ ★ ★ ★ Priorities for Personalized Medicine About the President’s Council of Advisors on Science and Technology President Bush established the President’s Council of Advisors on Science and Technology (PCAST) by Executive Order 13226 in September 2001. Under this Executive Order, PCAST “shall advise the President … on matters involving science and technology policy,” and “shall assist the National Science and Technology Council (NSTC) in securing private sector involvement in its activities.” The NSTC is a cabinet-level council that coordinates interagen- cy research and development activities and science and technology policy making processes across federal departments and agencies. PCAST enables the President to receive advice from the private sector, including the aca- demic community, on important issues relative to technology, scientific research, math and science education, and other topics of national concern. The PCAST-NSTC link provides a mechanism to enable the public-private exchange of ideas that inform the Federal science and technology policy making processes. As a private sector advisory committee, PCAST recommendations do not constitute Adminis- tration policy but rather advice to the Administration in the Science and Technology arena. PCAST follows a tradition of Presidential advisory panels on science and technology dating back to Presidents Eisenhower and Truman. The Council’s 35 members, appointed by the President, are drawn from industry, educational and research institutions, and other nongovernmental organizations. In addition, the Director of the Office of Science and Technology Policy serves as PCAST’s Co-Chair. ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ Priorities for Personalized Medicine Report of the President’s Council of Advisors on Science and Technology September 2008 ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ EXECUTIVE OFFICE OF THE PRESIDENT OFFICE OF SCIENCE AND TECHNOLOGY POLICY WASHINGTON, D.C. -

President Joseph R. Biden the White House 1600 Pennsylvania Avenue NW Washington, DC 20500
President Joseph R. Biden The White House 1600 Pennsylvania Avenue NW Washington, DC 20500 February 11, 2021 Dear President Biden, The undersigned physicians, researchers, and leaders in oncology, including representatives who served on your Cancer Moonshot Blue Ribbon Panel, would like to thank you for your lifelong commitment to science, patients, and innovation. As the First Lady said recently, “This is the fight of our lives” and we could not agree more. We stand ready to support you, Dr. Biden, and your Administration in your quest to end cancer as we know it. We applaud the inspiring appointments you have made to your cabinet and other leadership roles as you set out to combat cancer and COVID-19. We write to also express an urgency to make one of those remaining appointments and would like to respectfully voice our support for you to nominate Dr. Janet Woodcock as the permanent Commissioner of the U.S. Food and Drug Administration (FDA). As leaders in oncology with decades of experience, we know that now more than ever, the FDA requires a strong and respected leader who has the deep understanding of science, public health, and drug development to steer the country through these unprecedented challenges. Taking the next step and nominating Dr. Woodcock as FDA Commissioner is the leadership we need to move forward in our deep understanding of science and would affirm to the American public that the FDA will continue to be the global gold standard to change patient’s lives for the better. Dr. Woodcock is uniquely qualified to run the FDA at this vital moment for the science and research taking place across the country. -

Speaker Biographies
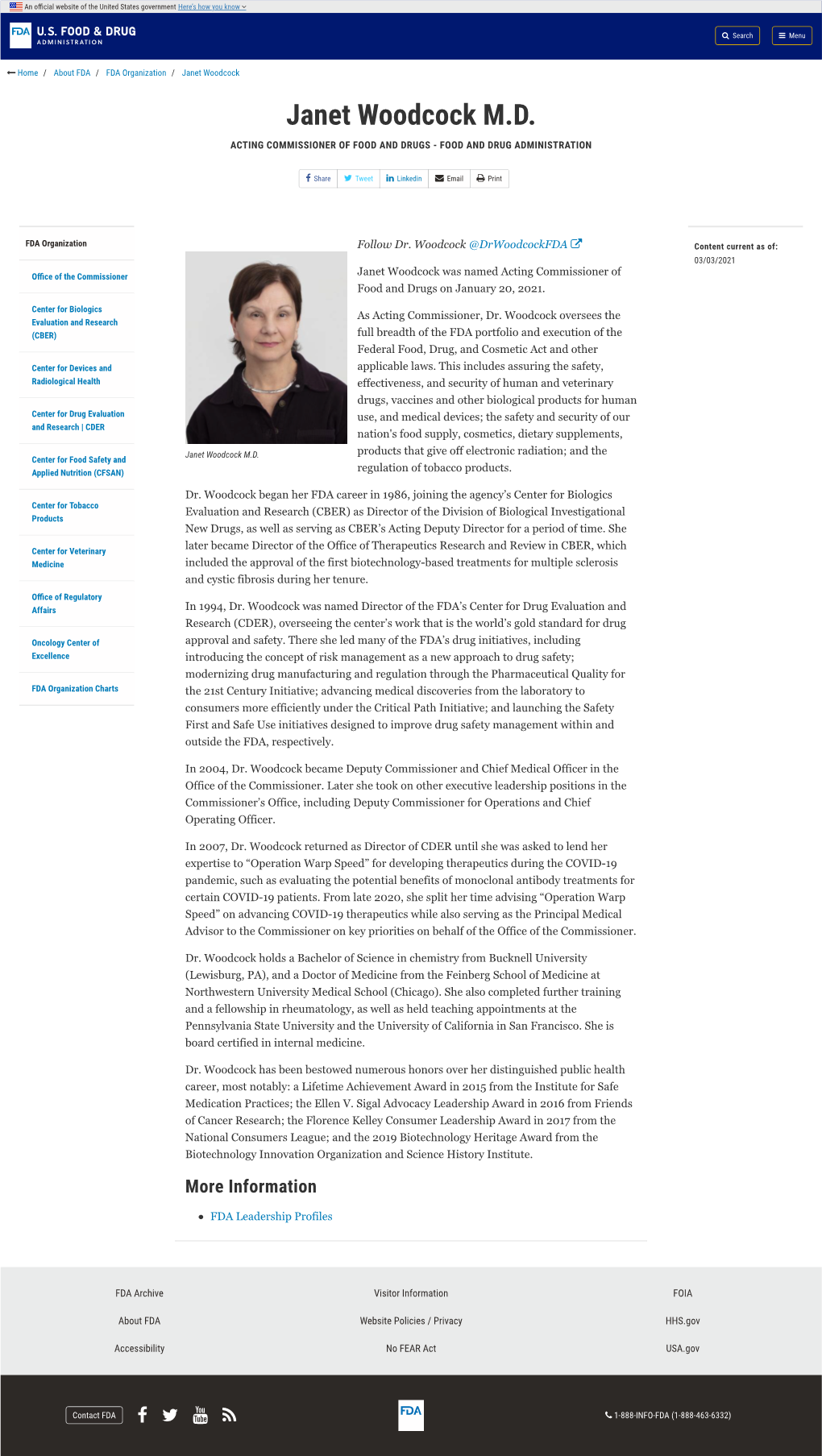
SPEAKER BIOGRAPHIES Keynote & Plenary Speakers Janet Woodcock, MD Acting Commissioner of Food and Drugs Food and Drug Administration (FDA) Janet Woodcock was named Acting Commissioner of Food and Drugs on January 20, 2021. As Acting Commissioner, Dr. Woodcock oversees the full breadth of the FDA portfolio and execution of the Federal Food, Drug, and Cosmetic Act and other applicable laws. This includes assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices; the safety and security of our nation's food supply, cosmetics, dietary supplements, products that give off electronic radiation; and the regulation of tobacco products. Dr. Woodcock began her FDA career in 1986, joining the agency’s Center for Biologics Evaluation and Research (CBER) as Director of the Division of Biological Investigational New Drugs, as well as serving as CBER’s Acting Deputy Director for a period of time. She later became Director of the Office of Therapeutics Research and Review in CBER, which included the approval of the first biotechnology-based treatments for multiple sclerosis and cystic fibrosis during her tenure. In 1994, Dr. Woodcock was named Director of the FDA’s Center for Drug Evaluation and Research (CDER), overseeing the center’s work that is the world’s gold standard for drug approval and safety. There she led many of the FDA’s drug initiatives, including introducing the concept of risk management as a new approach to drug safety; modernizing drug manufacturing and regulation through the Pharmaceutical Quality for the 21st Century Initiative; advancing medical discoveries from the laboratory to consumers more efficiently under the Critical Path Initiative; and launching the Safety First and Safe Use initiatives designed to improve drug safety management within and outside the FDA, respectively.