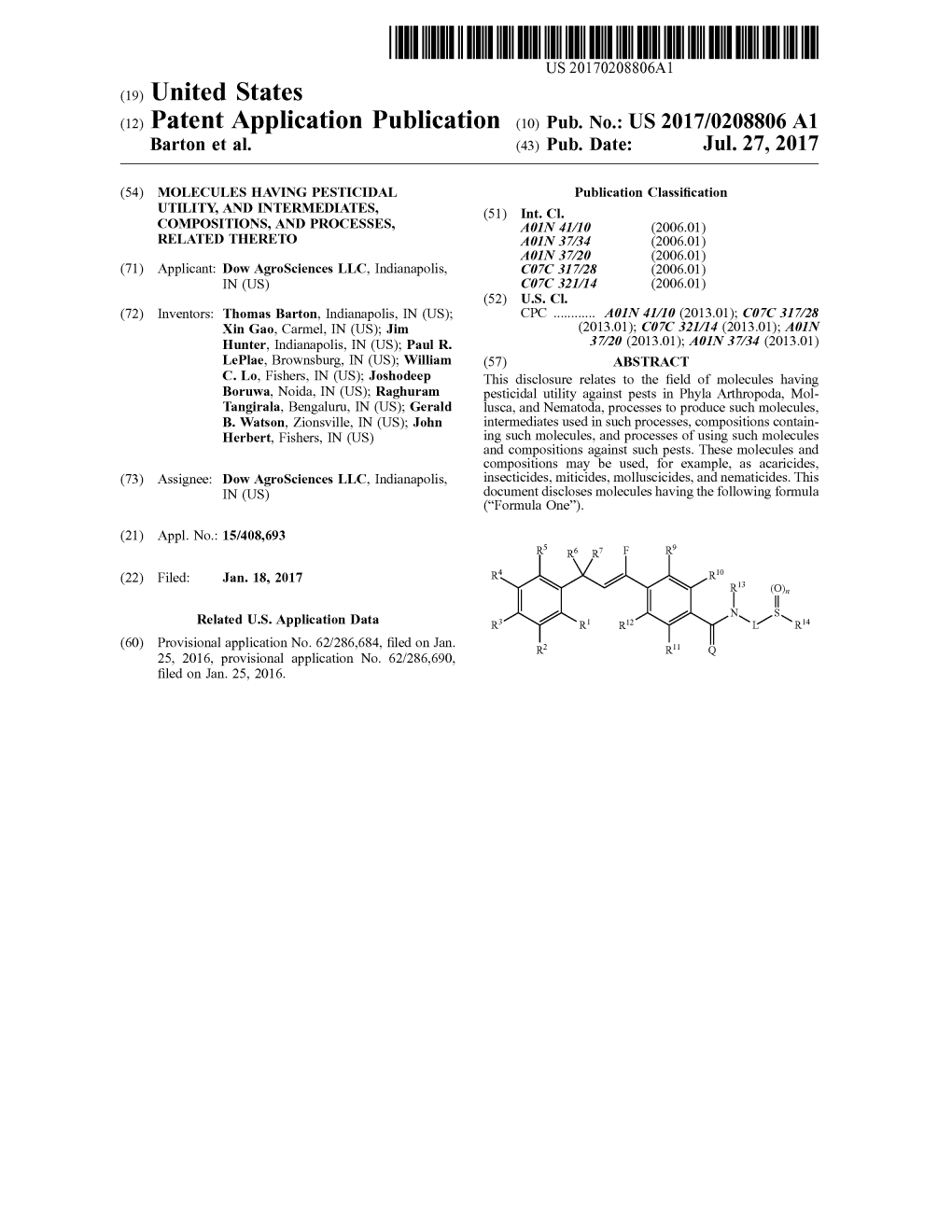
(12) Patent Application Publication (10) Pub. No.: US 2017/0208806 A1 Barton Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insert Report Title Here
Vegetable biosecurity & quarantine gap analysis Prue McMichael Scholefield Robinson Horticultural Services Pty Ltd Project Number: VG07087 VG07087 This report is published by Horticulture Australia Ltd to pass on information concerning horticultural research and development undertaken for the vegetable industry. The research contained in this report was funded by Horticulture Australia Ltd with the financial support of the vegetable industry. All expressions of opinion are not to be regarded as expressing the opinion of Horticulture Australia Ltd or any authority of the Australian Government. The Company and the Australian Government accept no responsibility for any of the opinions or the accuracy of the information contained in this report and readers should rely upon their own enquiries in making decisions concerning their own interests. ISBN 0 7341 1849 X Published and distributed by: Horticulture Australia Ltd Level 7 179 Elizabeth Street Sydney NSW 2000 Telephone: (02) 8295 2300 Fax: (02) 8295 2399 E-Mail: [email protected] © Copyright 2008 FINAL REPORT Vegetable Biosecurity and Quarantine Gap Analysis VG07087 Prepared for : Horticulture Australia Ltd HAL Project No. VG07087 Prepared by : Prue McMichael Completion Date : September 2008 SCHOLEFIELD ROBINSON HORTICULTURAL SERVICES PTY LTD 118A Glen Osmond Road, Parkside SA 5063 Australia ACN 008 199 737 PO Box 650, Fullarton SA 5063 Ph: (08) 8373 2488 ABN 63 008 199 737 Fax: (08) 8373 2442 Email: [email protected] Web Site: www.srhs.com.au Offices in Adelaide and Mildura Scholefield Robinson Horticultural Services Pty Ltd HAL Project No. VG 07087 PROJECT LEADER Dr Prue McMichael Senior Consultant/Plant Pathologist Scholefield Robinson Horticultural Services Pty Ltd PO Box 650 Fullarton SA 5063 PURPOSE OF REPORT This Final Report has been prepared to document information acquired, analysed and considered during the review undertaken for HAL, into all aspects of the biosecurity of Australia’s vegetable industries that are members of AUSVEG. -

Health Risk Assessment for the Introduction of Eastern Wild Turkeys (Meleagris Gallopavo Silvestris) Into Nova Scotia
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Canadian Cooperative Wildlife Health Centre: Wildlife Damage Management, Internet Center Newsletters & Publications for April 2004 Health risk assessment for the introduction of Eastern wild turkeys (Meleagris gallopavo silvestris) into Nova Scotia A.S. Neimanis F.A. Leighton Follow this and additional works at: https://digitalcommons.unl.edu/icwdmccwhcnews Part of the Environmental Sciences Commons Neimanis, A.S. and Leighton, F.A., "Health risk assessment for the introduction of Eastern wild turkeys (Meleagris gallopavo silvestris) into Nova Scotia" (2004). Canadian Cooperative Wildlife Health Centre: Newsletters & Publications. 48. https://digitalcommons.unl.edu/icwdmccwhcnews/48 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Canadian Cooperative Wildlife Health Centre: Newsletters & Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Health risk assessment for the introduction of Eastern wild turkeys (Meleagris gallopavo silvestris) into Nova Scotia A.S. Neimanis and F.A. Leighton 30 April 2004 Canadian Cooperative Wildlife Health Centre Department of Veterinary Pathology Western College of Veterinary Medicine 52 Campus Dr. University of Saskatchewan Saskatoon, SK Canada S7N 5B4 Tel: 306-966-7281 Fax: 306-966-7439 [email protected] [email protected] 1 SUMMARY This health risk assessment evaluates potential health risks associated with a proposed introduction of wild turkeys to the Annapolis Valley of Nova Scotia. The preferred source for the turkeys would be the Province of Ontario, but alternative sources include the northeastern United States from Minnesota eastward and Tennessee northward. -

Evaluation of Fluralaner and Afoxolaner Treatments to Control Flea
Dryden et al. Parasites & Vectors (2016) 9:365 DOI 10.1186/s13071-016-1654-7 RESEARCH Open Access Evaluation of fluralaner and afoxolaner treatments to control flea populations, reduce pruritus and minimize dermatologic lesions in naturally infested dogs in private residences in west central Florida USA Michael W. Dryden1*, Michael S. Canfield2, Kimberly Kalosy1, Amber Smith1, Lisa Crevoiserat1, Jennifer C. McGrady1, Kaitlin M. Foley1, Kathryn Green2, Chantelle Tebaldi2, Vicki Smith1, Tashina Bennett1, Kathleen Heaney3, Lisa Math3, Christine Royal3 and Fangshi Sun3 Abstract Background: A study was conducted to evaluate and compare the effectiveness of two different oral flea and tick products to control flea infestations, reduce pruritus and minimize dermatologic lesions over a 12 week period on naturally infested dogs in west central FL USA. Methods: Thirty-four dogs with natural flea infestations living in 17 homes were treated once with a fluralaner chew on study day 0. Another 27 dogs living in 17 different homes were treated orally with an afoxolaner chewable on day 0, once between days 28–30 and once again between days 54–60. All products were administered according to label directions by study investigators. Flea populations on pets were assessed using visual area counts and premise flea infestations were assessed using intermittent-light flea traps on days 0, 7, 14, 21, and once between days 28–30, 40–45, 54–60 and 82–86. Dermatologic assessments were conducted on day 0 and once monthly. Pruritus assessments were conducted by owners throughout the study. No concurrent treatments for existing skin disease (antibiotics, anti-inflammatories, anti-fungals) were allowed. -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

A Compilation and Analysis of Food Plants Utilization of Sri Lankan Butterfly Larvae (Papilionoidea)
MAJOR ARTICLE TAPROBANICA, ISSN 1800–427X. August, 2014. Vol. 06, No. 02: pp. 110–131, pls. 12, 13. © Research Center for Climate Change, University of Indonesia, Depok, Indonesia & Taprobanica Private Limited, Homagama, Sri Lanka http://www.sljol.info/index.php/tapro A COMPILATION AND ANALYSIS OF FOOD PLANTS UTILIZATION OF SRI LANKAN BUTTERFLY LARVAE (PAPILIONOIDEA) Section Editors: Jeffrey Miller & James L. Reveal Submitted: 08 Dec. 2013, Accepted: 15 Mar. 2014 H. D. Jayasinghe1,2, S. S. Rajapaksha1, C. de Alwis1 1Butterfly Conservation Society of Sri Lanka, 762/A, Yatihena, Malwana, Sri Lanka 2 E-mail: [email protected] Abstract Larval food plants (LFPs) of Sri Lankan butterflies are poorly documented in the historical literature and there is a great need to identify LFPs in conservation perspectives. Therefore, the current study was designed and carried out during the past decade. A list of LFPs for 207 butterfly species (Super family Papilionoidea) of Sri Lanka is presented based on local studies and includes 785 plant-butterfly combinations and 480 plant species. Many of these combinations are reported for the first time in Sri Lanka. The impact of introducing new plants on the dynamics of abundance and distribution of butterflies, the possibility of butterflies being pests on crops, and observations of LFPs of rare butterfly species, are discussed. This information is crucial for the conservation management of the butterfly fauna in Sri Lanka. Key words: conservation, crops, larval food plants (LFPs), pests, plant-butterfly combination. Introduction Butterflies go through complete metamorphosis 1949). As all herbivorous insects show some and have two stages of food consumtion. -

Molecular Basis of Pheromonogenesis Regulation in Moths
Chapter 8 Molecular Basis of Pheromonogenesis Regulation in Moths J. Joe Hull and Adrien Fónagy Abstract Sexual communication among the vast majority of moths typically involves the synthesis and release of species-specifc, multicomponent blends of sex pheromones (types of insect semiochemicals) by females. These compounds are then interpreted by conspecifc males as olfactory cues regarding female reproduc- tive readiness and assist in pinpointing the spatial location of emitting females. Studies by multiple groups using different model systems have shown that most sex pheromones are synthesized de novo from acetyl-CoA by functionally specialized cells that comprise the pheromone gland. Although signifcant progress was made in identifying pheromone components and elucidating their biosynthetic pathways, it wasn’t until the advent of modern molecular approaches and the increased avail- ability of genetic resources that a more complete understanding of the molecular basis underlying pheromonogenesis was developed. Pheromonogenesis is regulated by a neuropeptide termed Pheromone Biosynthesis Activating Neuropeptide (PBAN) that acts on a G protein-coupled receptor expressed at the surface of phero- mone gland cells. Activation of the PBAN receptor (PBANR) triggers a signal trans- duction cascade that utilizes an infux of extracellular Ca2+ to drive the concerted action of multiple enzymatic steps (i.e. chain-shortening, desaturation, and fatty acyl reduction) that generate the multicomponent pheromone blends specifc to each species. In this chapter, we provide a brief overview of moth sex pheromones before expanding on the molecular mechanisms regulating pheromonogenesis, and con- clude by highlighting recent developments in the literature that disrupt/exploit this critical pathway. J. J. Hull (*) USDA-ARS, US Arid Land Agricultural Research Center, Maricopa, AZ, USA e-mail: [email protected] A. -

Insect Survey of Four Longleaf Pine Preserves
A SURVEY OF THE MOTHS, BUTTERFLIES, AND GRASSHOPPERS OF FOUR NATURE CONSERVANCY PRESERVES IN SOUTHEASTERN NORTH CAROLINA Stephen P. Hall and Dale F. Schweitzer November 15, 1993 ABSTRACT Moths, butterflies, and grasshoppers were surveyed within four longleaf pine preserves owned by the North Carolina Nature Conservancy during the growing season of 1991 and 1992. Over 7,000 specimens (either collected or seen in the field) were identified, representing 512 different species and 28 families. Forty-one of these we consider to be distinctive of the two fire- maintained communities principally under investigation, the longleaf pine savannas and flatwoods. An additional 14 species we consider distinctive of the pocosins that occur in close association with the savannas and flatwoods. Twenty nine species appear to be rare enough to be included on the list of elements monitored by the North Carolina Natural Heritage Program (eight others in this category have been reported from one of these sites, the Green Swamp, but were not observed in this study). Two of the moths collected, Spartiniphaga carterae and Agrotis buchholzi, are currently candidates for federal listing as Threatened or Endangered species. Another species, Hemipachnobia s. subporphyrea, appears to be endemic to North Carolina and should also be considered for federal candidate status. With few exceptions, even the species that seem to be most closely associated with savannas and flatwoods show few direct defenses against fire, the primary force responsible for maintaining these communities. Instead, the majority of these insects probably survive within this region due to their ability to rapidly re-colonize recently burned areas from small, well-dispersed refugia. -

Complete Issue More Information About This Article Journal's Webpage in Redalyc.Org Basso, C.; Cibils-Stewart, X. Foundations An
Agrociencia Uruguay ISSN: 1510-0839 ISSN: 2301-1548 [email protected] Universidad de la República Uruguay Basso, C.; Cibils-Stewart, X. Foundations and developments of pest management in Uruguay: a review of the lessons and challenges Agrociencia Uruguay, vol. 24, no. 2, 2020, July-December, pp. 1-28 Universidad de la República Uruguay DOI: https://doi.org/10.31285/AGRO.24.409 Complete issue More information about this article Journal's webpage in redalyc.org Agrociencia Uruguay 2020 | Volume 24 | Number 2 | Article 409 DOI: 10.31285/AGRO.24.409 ISSN 2301-1548 Foundations and developments of pest management in Uruguay Editor a review of the lessons and challenges Martín Bollazzi Universidad de la República, Montevideo, Uruguay. Cimientos y desarrollo del manejo de Correspondence plagas en Uruguay César Basso, [email protected] una revisión de las lecciones y los desafíos Received 08 Sep 2020 Accepted 01 Oct 2020 Fundamentos e desenvolvimento do Published 13 Oct 2020 manejo de pragas no Uruguai Citation Basso C, Cibils-Stewart X. Foundations and developments uma revisão das lições e desafios of pest management in Uruguay: a review of the lessons and challenges. Agrociencia Uruguay [Internet]. Basso, C. 1; Cibils-Stewart, X. 2 2020 [cited dd mmm yyyy];24(2):409. Available from: http://agrocienciauruguay. uy/ojs/index.php/agrocien- 1Universidad de la República, Facultad de Agronomía, Unidad de cia/article/view/409 Entomología, Montevideo, Uruguay. 2Instituto Nacional de Investigación Agropecuaria (INIA), Programa Nacional de Investigación en Pasturas y Forrajeras, Entomología, Protección Vegetal, Colonia, Uruguay. History of pest management in Uruguay Abstract FAO has proclaimed 2020 as the “International Year of Plant Health”. -

ISSN 2320-5407 International Journal of Advanced Research (2015), Volume 3, Issue 1, 206-211
ISSN 2320-5407 International Journal of Advanced Research (2015), Volume 3, Issue 1, 206-211 Journal homepage: http://www.journalijar.com INTERNATIONAL JOURNAL OF ADVANCED RESEARCH RESEARCH ARTICLE BUTTERFLY SPECIES DIVERSITY AND ABUNDANCE IN MANIKKUNNUMALA FOREST OF WESTERN GHATS, INDIA. M. K. Nandakumar1, V.V. Sivan1, Jayesh P Joseph1, M. M. Jithin1, M. K. Ratheesh Narayanan2, N. Anilkumar1. 1 Community Agrobiodiversity Centre, M S Swaminathan Research Foundation,Puthoorvayal, Kalpetta, Kerala- 673121, India 2 Department of Botany, Payyanur College, Edat P.O., Kannur, Kerala-670327, India Manuscript Info Abstract Manuscript History: Butterflies, one of the most researched insect groups throughout the world, are also one of the groups that face serious threats of various kinds and in Received: 11 November 2014 Final Accepted: 26 December 2014 varying degrees. Wayanad district is one of the biodiversity rich landscapes Published Online: January 2015 within the biodiversity hot spot of Western Ghats. This paper essentially deals with the abundance and diversity of butterfly species in Key words: Manikkunnumala forest in Wayanad district of Western Ghats. The hilly ecosystem of this area is under various pressures mainly being Butterfly diversity, Abundance, anthropogenic. Still this area exhibits fairly good diversity; this includes Wayanad, Western Ghats some very rare and endemic butterflies. When assessed the rarity and *Corresponding Author abundance, six out of 94 recorded butterflies comes under the Indian Wildlife Protection Act, 1972. The area needs immediate attention to conserve the M. K. Nandakumar remaining vegetation in order to protect the butterfly diversity. Copy Right, IJAR, 2015,. All rights reserved INTRODUCTION Butterflies are one of the unique groups of insects, which grasp the attention of nature lovers worldwide. -

Arizona Department of Agriculture Environmental & Plant Services Division 1688 W
DOUGLAS A. DUCEY MARK W. KILLIAN Governor Director Arizona Department of Agriculture Environmental & Plant Services Division 1688 W. Adams Street, Phoenix, Arizona 85007 P. (602) 542-0994 F. (602) 542-1004 SUMMARY OF EXTERIOR QUARANTINES Updated April 16, 2021 CONTACTS Jack Peterson…...…………………………………………………………………………..…Associate Director (602) 542-3575 [email protected] Rachel Paul…………………………………………………………………………...Field Operations Manager (602) 542-3243 [email protected] Jamie Legg………………………………………………………………………..Quarantine Program Manager (602) 542-0992 [email protected] INDEX Summaries………………………………………………………………………………………...……….Page 2 Nursery Stock…………………………………………………………………………...…………Page 2 House Plants……………………………………………………………………………………….Page 2 Boll Weevil Pest…………………………………………………………………………………...Page 2 Citrus Nursery Stock Pests………………………………………………………………………...Page 3 Nut Tree Pests……………………………………………………………………………………..Page 3 Nut Pests…………………………………………………………………………………………...Page 4 Lettuce Mosaic Virus……………………………………………………………………………...Page 4 Imported Fire Ants………………………………………………………………………………...Page 5 Palm Tree Pests…………………………………………………………………………………....Page 5 Noxious Weeds…………………………………………………………………………………....Page 7 Japanese beetle…………………………………………………………………………………….Page 9 Arizona Administrative Code, Title 3, Chapter 4, Article 2 Quarantine……………………..………….Page 10 April 16, 2021 www.agriculture.az.gov Page 1 SUMMARIES Nursery Stock States Regulated - All states, districts, and territories of the United States. Regulated Commodities - All trees, shrubs, vines, cacti, agaves, succulents, -

Coleoptera Chrysomelidae) of Sicily: Recent Records and Updated Checklist
DOI: 10.1478/AAPP.982A7 AAPP j Atti della Accademia Peloritana dei Pericolanti Classe di Scienze Fisiche, Matematiche e Naturali ISSN 1825-1242 Vol. 98, No. 2, A7 (2020) THE CASSIDINAE AND CRYPTOCEPHALINI (COLEOPTERA CHRYSOMELIDAE) OF SICILY: RECENT RECORDS AND UPDATED CHECKLIST COSIMO BAVIERA a∗ AND DAVIDE SASSI b ABSTRACT. This paper compiles an updated checklist of the Sicilian Cassidinae and Cryptocephalini species (Coleoptera: Chrysomelidae, Cassidinae and Cryptocephalinae) starting from a critical bibliographic screening and adding new material, mainly collected by the first author in the last few decades. A total of 61 species is reported, withnew data for many rarely collected taxa. The provided data expand the known distribution of many uncommon species in Sicily. Two species are recorded for the first time: Cassida inopinata Sassi and Borowiec, 2006 and Cryptocephalus (Cryptocephalus) bimaculatus Fabricius, 1781 and two uncertain presences are confirmed: Cassida deflorata Suffrian, 1844 and Cassida nobilis Linné, 1758. The presence of other sixteen species is considered questionable and needs further confirmation. 1. Introduction Leaf beetles are all phytophagous Coleoptera. They are usually of a rather stout build, with a rounded or oval shape, often brightly coloured or with metallic hues. Worldwide some 32500 species in 2114 genera of Chrysomelidae have been described (Slipi´ nski´ et al. 2011), the majority of which occur in the tropics as is the case with numerous other Coleoptera families. Currently, the leaf beetle subfamily Cassidinae comprises the tortoise beetles (Cassidinae s. str.) and the hispine beetles (Hispinae s. str.) (Borowiec 1995; Hsiao and Windsor 1999; Chaboo 2007). So far, the Cassidinae list about 6300 described species within more than 340 genera, being the second most speciose subfamily of leaf beetles (Borowiec and Swi˛etoja´ nska´ 2014). -

NEXGARD SPECTRA, INN-Afoxolaner-Milbemycin Oxime
EMA/704200/2014 EMEA/V/C/003842 Nexgard Spectra (afoxolaner/milbemycin oxime) An overview of Nexgard Spectra and why it is authorised in the EU What is Nexgard Spectra and what is it used for? Nexgard Spectra is a veterinary medicine used to treat infestations with fleas, ticks, as well as demodectic and sarcoptic mange (skin infestations caused by two different types of mites) in dogs when prevention of heartworm disease (caused by a worm that infects the heart and blood vessels and is transmitted by mosquitoes), lungworm disease, eye worm and/or treatment of gut worms (hookworms, roundworms and whipworm) is also required. Nexgard Spectra contains the active substances afoxolaner and milbemycin oxime. How is Nexgard Spectra used? Nexgard Spectra is available as chewable tablets in five different strengths for use in dogs of different weights. It can only be obtained with a prescription. The appropriate strength of tablets should be used according to the dog’s weight. Treatment for fleas and ticks should be repeated at monthly intervals during the flea or tick seasons; Nexgard Spectra can be used as part of the seasonal treatment of fleas and ticks in dogs infected with gut worms. A single dose of Nexgard Spectra is given to treat gut worms. After which, further flea and tick treatment should be continued with a monovalent product containing a single active substance. For demodectic mange, treatment should be repeated monthly until the mange is successfully treated (as confirmed by two negative skin scrapings one month apart) whereas for sarcoptic mange treatment is given monthly for two months, or longer based on clinical signs and skin scrapings.