Energy Supply Device Aviation Rulemaking Committee
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Fire Apparatus Specifications
CLARE FIRE DEPARTMENT FIRE APPARATUS SPECIFICATIONS CUSTOM PUMPER 1 CLARE FIRE DEPARTMENT Y___N___ INTENT OF SPECIFICATIONS It is the intent of these specifications to cover the furnishing and delivery to the Clare Fire Department of a complete fire apparatus equipped as hereinafter specified. With the view of obtaining the best results and the most acceptable apparatus for service, these specifications cover only the general requirements as to the type of construction and tests to which the apparatus must conform, together with certain details to furnish equipment and appliances with which the successful bidder must conform. Minor details of construction and materials, where not otherwise specified, are left to the discretion of the contractor who shall be solely responsible for the design and construction of all features. The NATIONAL FIRE PROTECTION ASSOCIATION pamphlet #1901 current edition for Motor Vehicle Apparatus, unless otherwise specified in these specifications shall prevail. ONLY THE SPECIFIED FIREFIGHTING SUPPORT EQUIPMENT LISTED IN THESE SPECIFICATIONS SHALL BE PROVIDED. The apparatus shall conform to all Federal motor vehicle safety standards. Bids will only be considered from companies that have an established reputation in the field of fire and/or rescue apparatus manufacturing. Each bid must be accompanied by a set of detailed contractors specifications consisting of a detailed description of the apparatus and equipment proposed. These specifications shall include size, location, type, and model of all component parts being furnished. Detailed information shall be provided on the materials used to construct all facets of the apparatus body. Any bidder who fails to submit detailed construction specifications shall be considered non-responsive and shall render their proposal ineligible for award. -

Regulations and Standards for Clean Trucks and Buses on the Right Track?
CPB Corporate Partnership Board Regulations and Standards for Clean Trucks and Buses On the Right Track? Decarbonising Transport Regulations and Standards for Clean Trucks and Buses On the Right Track? Decarbonising Transport The International Transport Forum The International Transport Forum is an intergovernmental organisation with 62 member countries. It acts as a think tank for transport policy and organises the Annual Summit of transport ministers. ITF is the only global body that covers all transport modes. The ITF is politically autonomous and administratively integrated with the OECD. The ITF works for transport policies that improve peoples’ lives. Our mission is to foster a deeper understanding of the role of transport in economic growth, environmental sustainability and social inclusion and to raise the public profile of transport policy. The ITF organises global dialogue for better transport. We act as a platform for discussion and pre- negotiation of policy issues across all transport modes. We analyse trends, share knowledge and promote exchange among transport decision-makers and civil society. The ITF’s Annual Summit is the world’s largest gathering of transport ministers and the leading global platform for dialogue on transport policy. The Members of the Forum are: Albania, Armenia, Argentina, Australia, Austria, Azerbaijan, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Canada, Chile, China (People’s Republic of), Croatia, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, India, Ireland, Israel, Italy, Japan, Kazakhstan, Korea, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Mexico, Republic of Moldova, Mongolia, Montenegro, Morocco, the Netherlands, New Zealand, North Macedonia, Norway, Poland, Portugal, Romania, Russian Federation, Serbia, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Tunisia, Turkey, Ukraine, the United Arab Emirates, the United Kingdom, the United States and Uzbekistan. -

Supplier Quality Assurance Requirements (SQAR) Supplier Quality Assurance Manual Applies to RAM’S External Providers REV 5/20/2020
Supplier Quality Assurance Requirements (SQAR) Supplier Quality Assurance Manual Applies to RAM’s External Providers REV 5/20/2020 RAM COMPANY 3172 EAST DESERET DR SOUTH • ST GEORGE, UT 84790 SUPPLIER QUALITY ASSURANCE REQUIREMENTS (SQAR) SUPPLIER QUALITY MANUAL Applies to RAM’s External Providers Contents Q010 GENERAL REQUIREMENTS CLAUSES – Applies to all Purchase Orders .................................................................................. 6 1.0 PURPOSE ......................................................................................................................................................................................... 6 2.0 APPLICATION ................................................................................................................................................................................. 6 3.0 SUPPLIER RESPONSIBILITIES ..................................................................................................................................................... 6 3.1 Supplier Code of Conduct (Ethics and Safety) .............................................................................................................................. 6 3.1.1 Code of Conduct and Policy Enforcement ............................................................................................................................. 6 3.1.2 Confidentiality ...................................................................................................................................................................... -

Corrosion Resistance Enhancement of SAE 1020 Steel After Chromium Implantation by Nitrogen Ion Recoil 1. Introduction 2. Experim
Materials Research, Vol. 8, No. 4, 387-389, 2005 © 2005 Corrosion Resistance Enhancement of SAE 1020 Steel after Chromium Implantation by Nitrogen Ion Recoil Geraldo Francisco Gomesa*, Mario Uedab, Antonio Fernando Belotoc, Roberto Zenhei Nakazatod, Helfried Reuthere aScience and Technology Analyst, Laboratório de Combustão e Propulsão - LCP, Instituto Nacional de Pesquisas Espaciais, LCP - INPE, Rodovia Pres. Dutra, km 40, 12630-000 Cachoeira Paulista - SP, Brazil bLaboratório Associado de Plasma - LAP/INPE��������, Brazil cLaboratório Associado de Sensores e Materiais - LAS/INPE��������, Brazil dDepto. de Física e Química, UNESP/FEG, Guaratinguetá - SP��������, Brazil eForschungszentrum Rossendorf, FZR, Dresden, Germany Received: July 19, 2004; Revised: October 10, 2005 SAE 1020 construction steel is widely used as mortar reinforcement and small machine parts, but aside good surface properties as high ductility, hardness and wear resistance, its surface is prone to severe corrosion. As it is known, Chromium in amount over 12%-13% in the Fe alloys renders them resistance to several corrosive attacks. SAE 1020 samples were recovered with Chromium film and then bombarded either by nitrogen Ion Beam (IB) or Plasma Immersion Ion Implantation (PIII) to recoil implant Cr atoms in the Fe matrix. Samples treated by 100 keV N+ IB showed irregular, thin Cr profile, remaining a part of the film on the surface, to about 10 nm. Samples treated by 40 kV N PIII presented Cr layer of about 18% at., ranging to around 90 nm. Cr of the film was implanted in the Fe matrix in an almost flat profile. Results of corrosion test showed good performance of the PIII treated sample. -

Table of Contents From
Standards, Publications & Software Table of Contents from 34 Construction 89 Medical 110 Petroleum Global Engineering Documents®, the retail arm of IHS, is your single source for individual standards, 136 Telecommunications publications, and integrated collections via hardcopy or electronic delivery. Accessing critical technical 1 Aerospace/Aviation information has never been easier! 7 Annual Book of ASTM Standards global.ihs.com 17 Automotive/Heavy Equipment 25 Boiler & Pressure Vessel Code Copyright © 2004 by Information Handling Services Inc. All rights reserved. 30 CE Marking No portion of this material may be reprinted in any form without the experssed written permission of the publisher, Information Handling Services. Global Engineering Documents and logo are trademarks of Information Handling Services Inc. Registered U.S. Patent and Trademark Office. 32 Color Charts & Standards All other trademarks, brands and product names are the property of their respective owners. 41 Drawing & Drafting 46 Electrical/Electronics 67 Electromagnetic Compatibility/Frequency 70 Environmental 76 Fire Protection 79 Gears 82 Hardware 86 Hydraulics 104 Metals 108 Occupational Health and Safety 120 Plastics 124 Public Health 126 Quality 133 Safety 151 Transportation Management Systems 154 U.S. Government & Military 165 Welding 171 Timesaving Services Free Catalogs Available from Global . 171 Reference Materials & Services . 172 Standards Developing Organization Index . 174 Catalog Index . 177 PRIORITY CODE G040 To order or for more information: 800-854-7179 (USA/Canada) • fax: 303-397-2740 • global.ihs.com Aerospace/Aviation AV DATA® Aerospace Industries Association With AV-DATA® you have access to critical aviation information Global is the worldwide distributor of AIA standards and to help ensure worldwide regulatory compliance, airworthiness, publications. -

Guide to High Performance Alloys
Table of Contents Alloy Guide 3 The Alloys of Materion Brush Performance Alloys 4 Chemical Composition 6 Alloy Products 6 Physical Properties 8 Product Guide 10 Strip and Wire Temper Designations 11 Strip 12 Strip Product Specifications 13 Spring Bend Limits of Strip Alloys 19 Wire 20 Rod, Bar, Tube and Plate Temper Designations 22 Rod and Bar 22 Tube 26 Plate and Rolled Bar 29 MoldMAX® and PROtherm™ Plastic Tooling Alloys 31 ToughMet® and Alloy 25 Oilfield Products 32 Alloy 25 Drill String Temper (25 DST) 33 BrushCAST® Casting Ingot & Master Alloys 34 Forged and Extruded Finished Components 36 Cast Shapes 37 Recommended Replacements for Discontinued Alloys 38 Materion Brush Performance Alloys Value-Added Services 39 Custom Fabrication Capabilities 40 Customer Technical Service 40 Other Services 42 Processing and Fabrication Guide 43 Heat Treating Fundamentals 44 Forming of Strip 47 Cleaning and Finishing 50 Joining 51 Machining 53 Hardness 56 Microstructures 57 Other Attributes and Application Engineering Data 59 Fatigue Strength 60 Stress Relaxation Resistance 62 Corrosion Resistance 64 Magnetic Properties 67 Galling Resistance 68 Bearing Properties 69 Friction & Wear Properties 69 Resilience and Toughness 70 Elevated Temperature Properties 71 Cryogenic Properties 72 Other Attributes 72 Your Supplier 73 Company and Corporate History 74 Materion Corporate Profile 75 Brush Performance Alloys Mining & Manufacturing 77 Product Distribution 78 Customer Service 79 Quality 80 Health and Safety, Environment and Recycling 81 Alloy Guide The copper and nickel alloys commonly supplied in wrought product form are highlighted in this section. Wrought products are those in which final shape is achieved by working rather than by casting. -

Shotgun 199-213
BRILEY BROWNING CARLSON’S BROWNING INVECTOR DS SHOTGUN INDEX X2 SCREWIN CHOKE TUBE CHOKE TUBE Choke Tubes.................... 199-201 Replacement Parts.............. 203-204 Extended Length, Patterns Better Than Standard Competition Grade Tubes Choke Tubes Competition Gear.............. 210-212 Stocks........................ 207-210 SHOTGUN Improve Pattern & Accuracy Precision machined from 17-4 Magazine Tubes & Parts ........ 204-207 Tactical Accessories............. 212-213 Choke constrictions, from Cylin- stainless steel, these choke tubes fea- der to X-Full let you precisely match ture a 25% larger parallel section than conventional tubes, resulting Performance Parts.............. 201-202 your load to the shooting conditions. Long tube extends beyond the in tighter, denser, and more consistent patterns. Choke size is laser length of the muzzle, features highly polished interior for less pellet etched on each tube for easy identification, and the knurled end deformation; reduced fliers and tighter patterns. Knurled grasping makes removal quick and easy. Lead, copperplated, nickel Hevi- band speeds installation and removal. Choke constriction is marked Shot™, bismuth, tungsten, or steel shot may be used in these choke BIRCHWOOD CASEY on band, no need to remove tube to verify size. ab tubes. Do not use steel shot larger than BB in any Sporting Clays SCREWIN SPECS: 17-4 stainless steel. Polished, bright finish. Extends beyond muz- Choke Tube tighter than full constriction. ab CHOKE TUBE LUBE zle approx. .812" (20.6mm). Use with lead and bismuth shot. Steel shot SPECS: 17-4 stainless steel, polished. CHOKE ADAPTER can be used with constrictions of Modified and larger. Beretta chokes will Eliminates Stuck Choke Tubes, Barrel Damage STOCK # CHOKE DIA. -

Town of Guilford Request for Proposal Rfp #1-1718 Heavy Rescue Vehicle for the Fire Department
TOWN OF GUILFORD REQUEST FOR PROPOSAL RFP #1-1718 HEAVY RESCUE VEHICLE FOR THE FIRE DEPARTMENT CONTENTS I. Invitation to Bid (Legal Notice) __X__ II. General Conditions & Instructions to Bidders __X__ III. Fire Department Specifications and Required Submission by Vendors __X__ IV. Form Contract __X__ V. Non-Collusion/ Non-Conflict Affidavit (submit with proposal) __X__ VI. Affirmative Action Affidavit (submit with proposal) __X__ VII. Proposal Form (submit with proposal) __X__ REQUIREMENTS 1.) Certificate of Insurance _Yes__ Submit upon award 2.) Bid Bond/cashier’s check _Yes_ Include in bid proposal 3.) 100% Performance Bond _Yes_ Include in bid proposal 4.) Labor & Materials Bond _NO_ Not Applicable 5.) Vendor References _Yes__ Include in bid proposal 6.) Affidavits _Yes__ Include in bid proposal 7.) Required completion of questionnaire referenced in article 3 above _Yes__ Include in bid proposal RFP #1 - 1718 1 of 168 I. LEGAL NOTICE TOWN OF GUILFORD REQUEST FOR PROPOSAL #1 – 1718 HEAVY RESCUE VEHICLE FOR THE FIRE DEPARTMENT The Town of Guilford, on behalf of the Fire Department, is seeking competitive bids for a heavy-duty rescue vehicle for the Fire Department. Sealed proposals labeled “RFP #1-1718 Heavy Rescue Vehicle” and marked “time sensitive”, will be due no later than Friday December 8, 2017 at 2:00 p.m. at the office of the First Selectman, Town Hall Second Floor, 31 Park Street, Guilford CT 06437 at which time they will be opened publically. Proposals received after this date and time will be rejected. Request for Proposal packages may be downloaded from the Town of Guilford’s website at www.ci.guilford.ct.us and the Connecticut Department of Administrative Services Procurement website. -
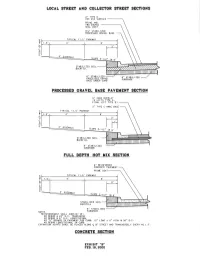
Final COV Specifications Ver 2007.2
TABLE OF CONTENTS CITY OF VICTORIA STANDARD SPECIFICATIONS TITLE DIVISION 01 Section 01010 - Summary of Work Section 01015 - Contractor Use of Premises Section 01025 - Measurement and Payment Section 01035 - Change Order Procedures Section 01040 - Coordination and Meetings Section 01045 - Cutting and Patching Section 01050 - Field Surveying Section 01090 - Reference Standards Section 01300 - Submittals Section 01380 - Construction Photographs Section 01410 - Testing Laboratory Services Section 01420 - Inspection Services Section 01500 - Temporary Facilities and Controls Section 01505 - Mobilization Section 01526 - Trench Safety System Section 01535 - Tree and Plant Protection Section 01563 - Control of Ground Water and Surface Water Section 01564 - Waste Material Disposal Section 01566 - Source Controls for Erosion and Sedimentation Section 01567 - Filter Fabric Fence Section 01568 - Reinforced Filter Fabric Barrier Section 01569 - Stabilized Construction Exit Section 01570 - Traffic Control and Regulation Section 01630 - Product Options and Substitutions Section 01700 - Contract Closeout Section 01720 - Project Record Documents DIVISION 02 Section 02100 - Right-of-Way Preparation Section 02221 - Embankment Section 02225 - Roadway Excavation Section 02227 - Excavation and Backfill for Utilities Section 02229 - Utility Backfill Materials Section 02230 – Proof Rolling Section 02231 - Crushed Stone Flexible Base Course Section 02233 - Cement Stabilized Base Course Section 02234 - Recycled Crushed Concrete Base Section 02235 - Flexible Base -

Cranfield University
Cranfield University G. James Sweers Methodology for the design assurance of aircraft lightning protection systems continued airworthiness. School of Engineering Aerospace Engineering Group PhD Thesis Academic Year 2009-2010 Supervisor: Professor J.P. Fielding September 2010 This thesis is submitted in partial fulfillment of the requirements for the Degree of Doctor of Philosophy. © Cranfield University, 2008. All rights reserved. No Part of this publication may be reproduced without the written permission of the copyright holder. Abstract This thesis describes a new approach to lightning protection components design by incorporating the use of multiple data sources, aircraft environmental threats models and component characteristics to determine if the component design meets the continued airworthiness requirements. This innovative aircraft lightning protection component design methodology examines critical component characteristics and evaluates these characteristics for long term survivability given known environmental design data. Use of in-service data, test data, material sciences and detailed component construction produces predictive results and provides inputs for the design community. A simple case of non-active lightning protection components was used to validate this methodology, concluding that certain design degradation mitigations are necessary to improve the continued airworthiness performance. Following this validation, the methodology was exercised by several case studies using actual design data from a large transport aircraft. The case studies provide for understanding how the methodology can be applied and showed that value was produced in creating design optimizations for the protection components. The case studies also proved that the methodology could be applied to different lightning protection designs spanning from structural design protection components to systems infrastructure transport elements and wiring. -

Heim Rod Ends and Sphericals
Rod Ends, Sphericals, Rolling Element Bearings The First Name in Rod EndsTM Heim® Bearings ISO 9001:2000 www.rbcbearings.com AS9100 800.390.3300 RBC Bearings has had a long tradition of innovation, commitment, and Heim® Rod Ends TABLE OF CONTENTS quality since the company was founded in 1919. Today, RBC Bearings has grown into a world-class manufacturer of standard and custom- Heim® Bearings produces the industry's widest range of rod end types Page No. engineered bearings and related products, with a product focus on and sizes (inch and metric). The Heim® Bearings product range research, testing, and development of the best product for specific includes rod ends with brass race inserts in standard, precision, and Rod Ends applications. high capacity designs; high strength two-piece designs; self-lubricating rod ends with engineered thermoplastic races or Teflon® liners; and mil- Selection Guide 4-5 What We Manufacture itary standard rod ends for the ultimate in rod end performance. Heim® rod ends are also available with a variety of platings, coatings, and HM, HF, HMX G, HFX G, BHM Precision 6-9 RBC Bearings, with facilities throughout North America and Europe, materials, and with a wide range of optional features such as lubrication HM C, HF C, M CR, F CR Commercial 10-13 provides bearings and precision products for applications in the con- fittings, left hand threads, and keyway slots. struction, mining, material handling, transportation and off-highway CMHD, CFHD Engineered Thermoplastic Race 14-15 equipment, robotics and automation, farming, machine tool, and semi- ROD ® ENDS conductor equipment industries. -

Ultrafine Grained Fe-Cr-Ni Austenitic Stainless Steels by Cold Rolling and Reversion Annealing: a Review of Progress in Iran
Naghizadeh M, J Ultrafine Grained Nanostruct Mater, 53(2), 2020, 210-223 Journal of Ultrafine Grained and Nanostructured Materials https://jufgnsm.ut.ac.ir Vol. 53, No.2, December 2020, pp. 210-223 Print ISSN: 2423-6845 Online ISSN: 2423-6837 DOI: 10.22059/jufgnsm.2020.02.13 Ultrafine grained Fe-Cr-Ni austenitic stainless steels by cold rolling and reversion annealing: A review of progress in Iran Meysam Naghizadeh, Mohammad Javad Sohrabi, Hamed Mirzadeh* School of Metallurgy and Materials Engineering, College of Engineering, University of Tehran, Tehran, Iran. Recieved: 5 September 2020; Accepted: 8 October 2020 * Corresponding author email: [email protected] ABSTRACT The notable contributions of Iranian scientists in the field of formation and reversion of strain-induced martensite for grain refinement and enhanced mechanical properties of Fe-Cr-Ni austenitic stainless steels are reviewed. Accordingly, the processing of ultrafine grained (UFG) structure via cold rolling and reversion annealing is summarized for AISI 301, 304, 309Si, 316, and 321 stainless steels, as well as their variants. The repetitive and innovative thermomechanical processing routes are introduced as well. The understanding of the stages of annealing (reversion of strain-induced martensite to austenite, primary recrystallization of the retained austenite, and grain growth) and the underlying reversion mechanisms (diffusional-type and martensitic shear-type) constitute the subsequent part of this overview. Finally, the transformation- induced plasticity (TRIP) effect in the reversion-treated metastable austenitic stainless steels is discussed. The present review paper summarizes these achievements with the discussion on the future prospects in this research field. Keywords: Austenitic stainless steels; Deformation-induced martensitic transformation; Reversion annealing; Ultrafine grained structure; Mechanical properties; TRIP effect.