Ecophysiology of Development: Hormones
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Perspectives of Forest Ecophysiological Research in the Context of Mediterranean Basin M
Invest Agrar: Sist Recur For (2005) 14(3), 538-549 Perspectives of forest ecophysiological research in the context of Mediterranean Basin M. Fernández* and R. Tapias Departamento de Ciencias Agroforestales. Escuela Politécnica Superior. Huelva University. 21819 Palos de la Frontera. Huelva. Spain Abstract The mediterranean-climate regions are linked to the presence of the unusual global conditions of cool rainy winters and warm dry summers. Thus, the common drought periods, frost nights, summer heat and fires and soil erosion disturb plants life. These regions have a global significance by their floristic richness. Nevertheless, these areas have been more heavily impacted by human activities than almost any other ecosystem. The foreseeable global change will most likely influence plant life, and the Mediterranean Basin will be specially affected (e.g. a decrease in spring and winter rainfalls, an increase in mean annual temperature), affecting the delicate equilibrium of these ecosystems. Ecophysiology is a powerful tool to examine the controlling mechanisms behind the functioning, distribution, abundance, and productivity of forest tree species and their response to a changing environment. This science discipline can help to the forest management by physiological approaches of the response of plant processes to the biotic and abiotic constraints imposed by the environment. Key words: Tree research, forest ecosystems, plant physiological ecology. Resumen Perspectivas en la investigación ecofisiológica forestal en el contexto de la cuenca mediterránea Las regiones de clima mediterráneo se caracterizan por presentar las precipitaciones concentradas en la época fría y veranos secos y calurosos. Ello conduce a la frecuente aparición de períodos de sequía, heladas, altas temperaturas estivales, fuegos forestales y fenómenos de erosión edáfica que afectan la vida de las plantas. -

Plant Ecophysiology – Biology 2210 (Or ES 2223)
Fall, 2019 Plant Ecophysiology – Biology 2210 (or ES 2223) Young Spruces - Rockwell Kent Professor Barry Logan Laboratory Instructor: Jaret Reblin Lecture: MWF: 10:40 – 11:35AM Laboratory: Tu, W or Th: 1:15 – 4:10PM Roux 207 Druckenmiller 222 Barry Logan Jaret Reblin Druckenmiller 220A Druckenmiller 222A 725-3944 725-3166 [email protected] [email protected] Office Hours: Tu. 9:15-10:25AM & W. 4:15-5:15PM M. 11:40 – 12:40PM & Th. 11:00 – 12:00PM (or by appointment) (or by appointment) Prerequisites: Biology 1102, 1109 or recommendation of the Biology Department Fall, 2019 Plant Ecophysiology – Biology 2210 (or ES 2223) Course Description: Examines the functional attributes of plants and the manner in which they vary across the plant kingdom by the processes of evolution and acclimation. Topics of focus include photosynthesis and protection against high-light stress, the acquisition and distribution of water and mineral nutrients, and environmental and hormonal control of development. Special topics discussed may include plant parasitism, carnivory, the origins and present state of agriculture, plant responses to global climate change, plant life in extreme environments, and the impacts of local land-use history on plant communities. Contemporary research instrumentation is used in weekly laboratories, some conducted in the field, to enable first-hand exploration of phenomena discussed in lecture. Inquiry in the Natural Sciences & Mathematical, Computational and Statistical Reasoning requirements: This course can be completed to satisfy either the INS or MCSR Distribution Requirement. This course is dedicated to understanding plant function, distribution and responses to the environment. We will pursue this through lecture; guided problem solving and critical thinking; reading and discussion of journal articles describing original research, review articles & chapters; field excursions; and field/lab exercises designed to offer you opportunities to pursue authentic research using contemporary instrumentation. -

Eleven Species of Liverworts As New Distributional Records to Bryoflora Andhra Pradesh, India
Bioscience Discovery, 11(3):111-120, July - 2020 © RUT Printer and Publisher Print & Online available on https://jbsd.in ISSN: 2229-3469 (Print); ISSN: 2231-024X (Online) Research Article Eleven species of liverworts as new distributional records to Bryoflora Andhra Pradesh, India. Ananthaneni Sreenath and Boyina Ravi Prasad Rao Biodiversity Conservation Division, Department of Botany, Sri Krishnadevaraya University, Ananthapuramu -515003, Andhra Pradesh. E-mail: [email protected] Article Info Abstract Received: 05-04-2020 Eleven species of liverworts viz., Asterella khasiana (Griff.) Grolle., Revised: 10-06-2020 Plagiochasma cordatum Lehm. & Lindenb., Plagiochasma intermedium Accepted: 18-06-2020 Lindend & Gottsche, Riccia frostii Austin, Riccia poihaniana A.E.D. Daniels Keywords: Eleven Species, & P. Daniel, Riccia sporocarpa Bisch. Riccia stricta (Gottsche, Lindenb. & Liverworts, New records, Nees) Perold, Riccia velimalaiana A.E.D. Daniels & P. Daniel, Andhra Pradesh Riccardia levieri Schiffner., Riccardia tenuicostata Schiffn. and Riccardia villosa (Stephani) S.C. Srivast. & Udar, are collected from different forest tracts of Eastern Ghats in Andhra Pradesh, are being new distributional records the state. INTRODUCTION: Plagiochasma intermedium Lindend & Gottsche, Andhra Pradesh is the seventh largest state Riccia frostii Austin, Riccia poihaniana A.E.D. in Indian union, it covers an area about 162, 970 sq. Daniels & P. Daniel, Riccia sporocarpa Bisch. kilometers and lies between 12°37ʹ and 19° 25ʹ Riccia stricta (Gottsche, Lindenb. & Nees) Perold, Northern latitude and 76° 45ʹ and 84° 72ʹ Eastern Riccia velimalaiana A.E.D. Daniels & P. Daniel, longitude (Map 1). The state comprises 13 districts, Riccardia levieri Schiffner., Riccardia tenuicostata there are two areas namely called Rayalaseema and Schiffn. and Riccardia villosa (Stephani) S.C. -

(Vell.) Mart. (Mimosaceae) Seeds Subjected to Hypoxia and Anoxia1
Revta brasil. Bot., São Paulo, V.23, n.1, p.51-57, mar. 2000 Ecophysiology and respiratory metabolism during the germination of Inga sessilis (Vell.) Mart. (Mimosaceae) seeds subjected to hypoxia and anoxia1 JANETE MAYUMI OKAMOTO2 and CARLOS ALFREDO JOLY2,3 (received: May 27, 1999; accepted: November 17, 1999) ABSTRACT - (Ecophysiology and respiratory metabolism during the germination of Inga sessilis (Vell.) Mart. (Mimosaceae) seeds subjected to hypoxia and anoxia). This paper presents a study on the respiratory metabolism of germinating seeds of Inga sessilis subjected to normoxia, hypoxia and anoxia. Although it is typical of environments where waterlogging seldom occurs, 40% of its seeds are able to germinate under hypoxia; yet, anoxia periods over 96 h are lethal to the seeds. Ethanol is the main product of the seeds anaerobic metabolism, but the steep increase in lactate after 24 h anoxia or 48 h hypoxia may explain the drop in seed viability. RESUMO - (Ecofisiologia da germinação e metabolismo respiratório de sementes de Inga sessilis (Vell.) Mart. (Mimosaceae) submetidas à hipoxia e anoxia). Os estudos referentes à germinação e ao metabolismo respiratório de sementes de Inga sessilis (Vell.) Mart. submetidas a normoxia, hipoxia e anoxia, mostraram que apesar de ser uma espécie típica de ambientes com solos bem drenados, 40% das sementes germinam sob hipoxia. A anoxia, por outro lado, é letal para as sementes. O etanol é o principal produto do metabolismo anaeróbico das sementes. A perda de viabilidade das sementes parece estar associada ao rápido aumento na concentração de lactato após 24 h de anoxia ou 48 h de hipoxia. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

2.10 Meesia Longiseta HEDW. Code: 1389 Anhang: II
2.10 Meesia longiseta HEDW. Code: 1389 Anhang: II KLAUS WEDDELING, GERHARD LUDWIG & MONIKA HACHTEL, Bonn Namen: D: Langstieliges Schwanenhalsmoos, Langstieliges Meesemoos, Gestreckte Langborste E: Long-stalked Thread Moss, Long-shafted Swan Moss, F: – Systematik/Taxonomie: Bryophyta, Bryopsida, Bryidae, Splachnales, Meesiaceae. Synonyme: Amblyodon longisetus (HEDW.) P. BEAUV. Kennzeichen/Artbestimmung: Meesia longiseta ist ein 4–8 (–10) cm hohes, akrokarpes, unverzweigtes Laubmoos von grün-schwärzlicher Färbung. Die Art wächst in lockeren, weichen Rasen (Abb. 2.9). Das Stämmchen ist bis in die Spitze wurzelhaarig und im Moose Querschnitt dreikantig. Die Blättchen sind mehr oder weniger deutlich in 3 oder 6 Rei- hen angeordnet und vom Stämmchen abgespreizt. Die 2–3,5 mm langen, spitzen Blätt- chen laufen deutlich am Stämmchen herab, sind oberwärts gekielt, ganzrandig oder an der Spitze etwas gezähnt. Ihre deutlich entwickelte Rippe endet unterhalb der Blattspitze. Der Blattrand ist flach. Die Laminazellen sind rechteckig bis rhombisch und etwa 14 µm breit. Die rötlichen, gedrehten Seten der synözischen Art können über 10 cm lang wer- den. Die langbirnenförmige, aufrechte Kapsel hat einen deutlichen Hals. Bei der Spo- renreife im Juni und Juli werden die mit 36–44 µm Durchmesser recht großen Sporen frei- gesetzt. Die Chromosomenzahl ist nicht bekannt (FRITSCH 1991). Differenzierende Merkmale zu den ähnlichen Arten Meesia uliginosa und M. hexasticha sind der nicht ein- gerollte Blattrand, die kleineren Sporen und der Rippenquerschnitt mit kleinen, inneren Zellen (zusammengestellt nach CRUM & ANDERSON 1981, FRAHM 1979, LIMPRICHT 1895). Abbildungen der Art finden sich bei CRUM & ANDERSON (1981, Fig. 296, 297, S. 628, 629: Blättchen, Blattspitze, Habitus, Kapsel) und FRAHM (1979, Fig. -

The MADS-Domain Protein PPM2 Preferentially Occurs in Gametangia
Gene 400 (2007) 25–34 www.elsevier.com/locate/gene The MADS-domain protein PPM2 preferentially occurs in gametangia and sporophytes of the moss Physcomitrella patens ⁎ Vanessa Quodt, Wolfram Faigl, Heinz Saedler, Thomas Münster Max Planck Institute for Plant Breeding Research, Department of Molecular Plant Genetics, Cologne, Germany Received 4 April 2007; received in revised form 23 May 2007; accepted 24 May 2007 Available online 5 June 2007 Received by W. Martin Abstract To date, the function of MADS-domain transcription factors in non-seed plants remains largely elusive, although a number of genes have been isolated and characterized from a variety of species. In our study we analyzed PPM2, a classical MIKC-type MADS-box gene from the moss Physcomitrella patens, taking advantage of the unique technical properties Physcomitrella offers in terms of efficient homologous recombination. We determined mRNA and protein distribution and performed targeted disruption of the genomic locus for functional analysis of PPM2. Despite weak ubiquitous expression, PPM2 protein is mostly found in male and female gametangia and basal parts of developing sporophytes. Therefore, PPM2 seems to function in both the haploid and the diploid phase of the moss life cycle. This situation reflects an evolutionary transition state of gene recruitment from an ancestral gametophytic generation into a derived sporophytic generation which became dominating in tracheophytes. However, a knock-out of the PPM2 gene did not cause visible phenotypical changes in the respective structures. The implications of our findings for the understanding of the evolutionary history of MADS-box transcription factors in plants are discussed. © 2007 Elsevier B.V. -

Forage Plant Ecophysiology: a Discipline Come of Age
agriculture Editorial Forage Plant Ecophysiology: A Discipline Come of Age Cory Matthew 1,* and Lilian Elgalise Techio Pereira 2 1 Institute of Agriculture and Environment PN 433, Massey University, Private Bag 11-222, Palmerston North 4442, New Zealand 2 Animal Science Department, University of São Paulo, Faculdade de Zootecnia e Engenharia de Alimentos, Av. Duque de Caxias Norte, 225, Pirassununga CEP 13635-900, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +64-6-356-9099 (ext. 84802); Fax: +64-6-350-5679 Received: 17 July 2017; Accepted: 24 July 2017; Published: 27 July 2017 1. Introduction The first use of the term “ecology” is credited to German scientist Ernst Haekel in 1866, who used the word to describe the total science of relationships between organisms and their environment [1]. Over time, the complexity of organism-environment interactions has led to the definition of specialist fields within the wider discipline of ecology, one of those being ‘ecophysiology’. The dictionary definition of ecophysiology is, “the science of the relationships between the physiology of organisms and their environment” [2]. The first use of the term ‘ecophysiology’ known to the authors was in 1956, by a French entomologist employed by L’Institut National de la Recherche Agronomique (INRA), Remy Chauvin [3]. Credit for forward thinking should be given to the staff of INRA, who in the mid-1980s established a centre at Lusignan initially known as the Station d’Ecophysiologie des Plantes Fourragères (SEPF), and later as the Unité d’Ecophysiologie des Plantes Fourragères (UEPF). In 2008, the UEPF was incorporated into the Unité de Recherche Pluridiciplinaire Prairies et Plantes Fourragères (URP3F) [4]. -

Action C.5 Milestone: Bryophyte Ex Situ Conservation Scheme Feb 2017
ESCAPE – UNIVERSITY OF HELSINKI Action C.5 Milestone Bryophyte ex situ conservation scheme Sanna Laaka-Lindberg & Xiaolan He 2/28/2017 Monitoring Meesia longiseta reintroduction site in 2016. Photo: Sanna Laaka-Lindberg An ex situ conservation scheme is presented on the basis of compiled results of the ESCAPE project as a model for bryophyte conservation. ESCAPE LIFE+2011 BIO/FI/917 Action C.5 Milestone Bryophyte ex situ conservation scheme Introduction Ex situ conservation is a species conservation method used as a compliment to the primary conservation tool in situ conservation, a process of protecting an endangered species in its natural habitat. Ex situ conservation is targeted to species in most serious threat, especially when the conservation measures in nature are not adequate for species survival. There are basically two different types of ex situ conservation tools: 1) tools aimed at storing and securing the species and its genetic variation e.g. on national or even on wider (global) level, and 2) tools aimed at increasing species survival ability in nature. The ultimate goal of ex situ conservation is to provide support for the survival of species in their natural environments. Conservation of biodiversity is a continuous and long-term assignment, so the decisions on ex situ conservation to a species need to be made on a solid basis. This involves thorough investigation on conservation priorities, background knowledge of the biology and ecology of the species to be conserved, and the feasibility of the conservation plan. In the ESCAPE project, a priority list was compiled for vascular plants (see Ryttäri 2013), but no such list is made for bryophytes. -
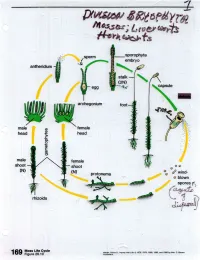
02-Bryophyta-2.Pdf
498 INTRODUCTORY PLANT SCIENCE layer. In certain species, the capsule con til1Ues to grow as long as the gametophyte lives. The presence of a meristcm in A11tlioceros and an aerating system complete with sto mata may indicate that Antlwceros evolved from ancestors with even ·larger· and more 1. Civ complex sporophytes. These features may Bryophyt be vestiges from a more complex ancestral. 2. Wh sporophyte. that ti alga ? 3. De� ORIGIN AND RELATIONSHIPS OF THE Bryophyt, BRYOPHYTA 4._ characteri Little is known with certainty about the r are small origin and evolution of the bryophytes. The ' tall. ( B) fossil record is too fragmenta to enable • ry s true roots us to trace their evolutionary history. Frag ,, sperms ar mentary remnants of thallose liverworts, spore idium and mother\4'/ttN) which resemble present-day liverworts, have cell Jar arch� been foum! in rocks of Carboniferous age, bryophyt as have structures that may be remains of more com mosses. all bryoph The immediate ancestors of the bryo bryo whicl phytes were probably more complex plants from the have an i than present-day forms. In other words, the gametophy evolutionary tendency has been one of re- with a dip1 duction instead of increased complexity. •• • • • spores If evolution has progressed . from more • . •, .... • s. __ complex sporophytes to those of simpler • . vascular p Anthoceros . form, the sporophyte of wou1d • • green algat be considered more ancient than that of • (D) red Marchantia or Riccia. Because Riccia has Fig. 35-16. longitudinal section of the spo 6. How the most reduced sporophyte, it would be t·:i;,hyte of Anthoceros. -

Creating Ponds for Rare Mosses and Liverworts
Creating ponds for rare mosses and liverworts Freshwater Habitats Trust 1. Mosses and liverworts Key messages Mosses and liverworts, collectively known as bryophytes, are an incredibly • Clean water is essential for diverse group of plants. There are over 1,000 species in the UK occurring all of our rarest mosses and in almost every habitat, from dappled shade in woodlands to almost bare liverworts. Avoid areas where limestone crags. Within these habitats the margins of ponds, lakes and the adjacent landuse could pools provide an important resource for many species because they add nutrients or pollution to provide areas of bare wet mud on which bryophytes can germinate surface waters or (Figure 1). groundwater. Unfortunately due to habitat loss, regulation of water levels and declines • On mineral or forestry sites, in the availability of clean unpolluted water, many bryophytes are now ensure that waterbodies have seriously threatened (Figure 2). By creating suitable pond habitats we can bryophyte friendly after uses. give rare mosses and liverworts the best chance of recovery. If needed, partition the site into areas for recreation and those for wildlife conservation. • Ensure that all waterbodies whether large or small have very wide shallow margins. Freshwater Habitats Trust This will increase the width of the drawdown zone and © Michael Lüth © Michael © David Holyoak © David Holyoak optimise the area available Figure 1. Bryophytes growing on bare mud within the pond margin: for bryophytes. Norfolk Bladder-moss Physcomitrium eurystomum (left) and Lizard • Create a complex of ponds of Crystalwort Riccia bifurca (right). different sizes. This will 2. Designing ponds for bryophytes provide a range of different environmental conditions and The spores of mosses and liverworts can readily move to new ponds on support the greatest number the feet of grazing animals and wildfowl.