3.4 Molecules & Colour
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aromaticity Sem- Ii
AROMATICITY SEM- II In 1931, German chemist and physicist Sir Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties .This is a very popular and useful rule to identify aromaticity in monocyclic conjugated compound. According to which a planar monocyclic conjugated system having ( 4n +2) delocalised (where, n = 0, 1, 2, .....) electrons are known as aromatic compound . For example: Benzene, Naphthalene, Furan, Pyrrole etc. Criteria for Aromaticity 1) The molecule is cyclic (a ring of atoms) 2) The molecule is planar (all atoms in the molecule lie in the same plane) 3) The molecule is fully conjugated (p orbitals at every atom in the ring) 4) The molecule has 4n+2 π electrons (n=0 or any positive integer Why 4n+2π Electrons? According to Hückel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons. - This is true of aromatic compounds, meaning they are quite stable. - With aromatic compounds, 2 electrons fill the lowest energy molecular orbital, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is denoted by n), leaving all bonding orbitals filled and no anti-bonding orbitals occupied. This gives a total of 4n+2π electrons. - As for example: Benzene has 6π electrons. Its first 2π electrons fill the lowest energy orbital, and it has 4π electrons remaining. These 4 fill in the orbitals of the succeeding energy level. The criteria for Antiaromaticity are as follows: 1) The molecule must be cyclic and completely conjugated 2) The molecule must be planar. -

AROMATIC COMPOUNDS Aromaticity
AROMATICITY AROMATIC COMPOUNDS Aromaticity Benzene - C6H6 H H H H H H H H H H H H Kekulé and the Structure of Benzene Kekule benzene: two forms are in rapid equilibrium 154 pm 134 pm • All bonds are 140 pm (intermediate between C-C and C=C) • C–C–C bond angles are 120° • Structure is planar, hexagonal A Resonance Picture of Bonding in Benzene resonance hybrid 6 -electron delocalized over 6 carbon atoms The Stability of Benzene Aromaticity: cyclic conjugated organic compounds such as benzene, exhibit special stability due to resonance delocalization of -electrons. Heats of hydrogenation + H2 + 120 KJ/mol + 2 H2 + 230 KJ/mol calc'd value= 240 KJ/mol 10 KJ/mol added stability + 3 H 2 + 208 KJ/mol calc'd value= 360 KJ/mol 152 KJ/mol added stability + 3 H2 + 337 KJ/mol 1,3,5-Hexatriene - conjugated but not cyclic Resonance energy of benzene is 129 - 152 KJ/mol An Orbital Hybridization View of Bonding in Benzene • Benzene is a planar, hexagonal cyclic hydrocarbon • The C–C–C bond angles are 120° = sp2 hybridized • Each carbon possesses an unhybridized p-orbital, which makes up the conjugated -system. • The six -electrons are delocalized through the -system The Molecular Orbitals of Benzene - the aromatic system of benzene consists of six p-orbitals (atomic orbitals). Benzene must have six molecular orbitals. Y6 Y4 Y5 six p-orbitals Y2 Y3 Y1 Degenerate orbitals: Y : zero nodes orbitals that have the 1 Bonding same energy Y2 and Y3: one node Y and Y : two nodes 4 5 Anti-bonding Y6: three node Substituted Derivatives of Benzene and Their Nomenclature -

Bsc Chemistry
Subject Chemistry Paper No and Title Paper 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry) Module No and Module 3: Hyper-Conjugation Title Module Tag CHE_P1_M3 CHEMISTRY PAPER No. 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry) Module No. 3: Hyper-Conjugation TABLE OF CONTENT 1. Learning outcomes 2. Introduction 3. Hyperconjugation 4. Requirements for Hyperconjugation 5. Consequences and Applications of Hyperconjugation 6. Reverse Hyperconjugation 7. Summary CHEMISTRY PAPER No. 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry) Module No. 3: Hyper-Conjugation 1. Learning Outcomes After studying this module you shall be able to: Understand the concept of hyperconjugation. Know about the structural requirements in a molecule to show hyperconjugation. Learn about the important consequences and applications of hyperconjugation. Comprehend the concept of reverse hyperconjugation. 2. Introduction In conjugation, we have studied that the electrons move from one p orbital to other which are aligned in parallel planes. Is it possible for electron to jump from p orbital to sp3 orbital that are not parallelly aligned with one another? The answer is yes. This type of conjugation is not normal, it is extra-ordinary. Hence, the name hyper-conjugation. It is also know as no-bond resonance. Let us study more about it. 3. Hyperconjugation The normal electron releasing inductive effect (+I effect) of alkyl groups is in the following order: But it was observed by Baker and Nathan that in conjugated system, the attachment of alkyl groups reverse their capability of electron releasing. They suggested that alkyl groups are capable of releasing electrons by some process other than inductive. -

Sc-Homoaromaticity
This is a repository copy of Modern Valence-Bond Description of Homoaromaticity. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/106288/ Version: Accepted Version Article: Karadakov, Peter Borislavov orcid.org/0000-0002-2673-6804 and Cooper, David L. (2016) Modern Valence-Bond Description of Homoaromaticity. Journal of Physical Chemistry A. pp. 8769-8779. ISSN 1089-5639 https://doi.org/10.1021/acs.jpca.6b09426 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Modern Valence-Bond Description of Homoaromaticity Peter B. Karadakov; and David L. Cooper; Department of Chemistry, University of York, Heslington, York, YO10 5DD, U.K. Department of Chemistry, University of Liverpool, Liverpool L69 7ZD, U.K. Abstract Spin-coupled (SC) theory is used to obtain modern valence-bond (VB) descriptions of the electronic structures of local minimum and transition state geometries of three species that have been con- C sidered to exhibit homoconjugation and homoaromaticity: the homotropenylium ion, C8H9 , the C cycloheptatriene neutral ring, C7H8, and the 1,3-bishomotropenylium ion, C9H11. -

Conjugated Molecules
Conjugated Molecules - Conjugated molecules have alternating single and multiple (i.e. double or triple) bonds. Example 1: Nomenclature: 2,6-dimethylhepta-2,5-diene This molecule is not conjugated because it does not have alternating single and multiple bonds (the arrangement of the bonds starting from C2 is double, single, single and double). Between the two double bonds, there is a saturated center (C4) and two intersecting single bonds. Example 2: Nomenclature: 2,5-dimethylhexa-2,4-diene This molecule is conjugated because the arrangement of the bonds starting at C2 is double, single, and double. They are alternated. Example 3: Nomenclature: (2E)-hept-2-en-5-yne note: (2E) is a stereochemical identifier. The letter “E” indicates the arrangement of the double bond (where E usually refers to trans and Z refers to cis). The number “2” indicates the position of the double bond. This molecule is not conjugated because the single and multiple bonds are not alternated. Example 4: Nomenclature: (2Z)-hex-2-en-4-yne This molecule is conjugated because there is an alternation of multiple and single bonds (double, single, and triple starting from C2). More examples: note: the term conjugation refers to parts of the molecule. If you can find one conjugated system within the molecule, that molecule is said to be conjugated. Example: In this molecule, the double bond A is not conjugated. However, since double bond B is conjugated with double bond C, the molecule is said to be conjugated. Special Nomenclature: The Letter "S" stands for “single” and indicates that we are talking about the conjugated double bonds. -

Organic and Biological Chemistry
CHAPTER 23 Organic and Biological Chemistry CONTENTS HO H 23.1 ▶ Organic Molecules and Their C Structures: Alkanes H H C 23.2 ▶ Families of Organic Compounds: HO O O C C Functional Groups 23.3 ▶ Naming Organic Compounds H CC 23.4 ▶ Carbohydrates:HO A Biological Example HO OH of Isomers H 23.5 ▶ Valence Bond TCheory and Orbital OverlapH Pictures H C 23.6 ▶ Lipids:HO A Biological EOxample ofO Cis–Trans IsomerismC C 23.7 ▶ Formal Charge and Resonance in Organic CompoundsH CC 23.8 ▶ Conjugated SystemsHO OH 23.9 ▶ Proteins: A Biological Example of Conjugation 23.10 ▶ Aromatic Compounds and Molecular Ascorbic acid, also known as vitamin C, is an essential nutrient in the human diet Orbital Theory because it is not synthesized in our body. We can eat citrus fruits or take pills that contain vitamin C to maintain health. 23.11 ▶ Nucleic Acids: A Biological Example of Aromaticity ? Which Is Better, Natural or Synthetic? The answer to this question can be found in the INQUIRY ▶▶▶ on page 1021. STUDY GUIDE M23_MCMU3170_07_SE_C23.indd 978 27/11/14 2:11 AM f the ultimate goal of chemistry is to understand the world around us on a molecular level, then a knowledge of biochemistry—the chemistry of living organisms—is a central part Iof that goal. Biochemistry, in turn, is a branch of organic chemistry, a term originally used to mean the study of compounds from living organisms while inorganic chemistry was used for the study of compounds from nonliving sources. Today, however, we know that there are no fundamental differences between organic and inorganic compounds; the same principles apply to both. -

Chapter 12 Arenes and Aromaticity
CH. 12 Chapter 12 Arenes and Aromaticity Arenes Arenes are hydrocarbon derivatives of benzene. They are called aromatic systems due to their special stability (not due to their aroma!). The special stability results from the highly conjugated system with the electrons delocalized all the way around the ring. The name benzene comes from the Arabic luban jawi or “incense from Java” since it is isolated as a degradation product of gum benzoin. This is a balsam obtained from a tree that grows in Java and Sumatra. This degradation product is benzoic acid, which can be decarboxylated by heating with calcium oxide, CaO. O C OH CaO + CO2 heat benzoic acid benzene And tolu balsam from the South American tolu tree, when distilled, produces toluene or methylbenzene. The term aliphatic hydrocarbons, given to alkanes, alkenes, alkynes and benzene derivatives, comes from the Greek word aleiphar which means oil or unguent. Benzene Benzene has three double bonds that are conjugated and in a circular arrangement. Due to this conjugation and circular arrangement, the double bonds are much less reactive than normal, isolated alkenes. For example, benzene will not react with bromine or hydrogen gas in the presence of a metal catalyst, reactions that alkenes undergo readily. Br H Br2 CH3 CH CH CH3 CH3 C C CH3 rt H Br Br2 No Reaction rt 1 CH. 12 H2, Pd CH3 CH CH CH3 CH3CH2CH2CH3 rt H , Pd 2 No Reaction rt If we examine the structure of benzene we do not see a series of alternating double and single bonds as we would expect from the Lewis structure. -

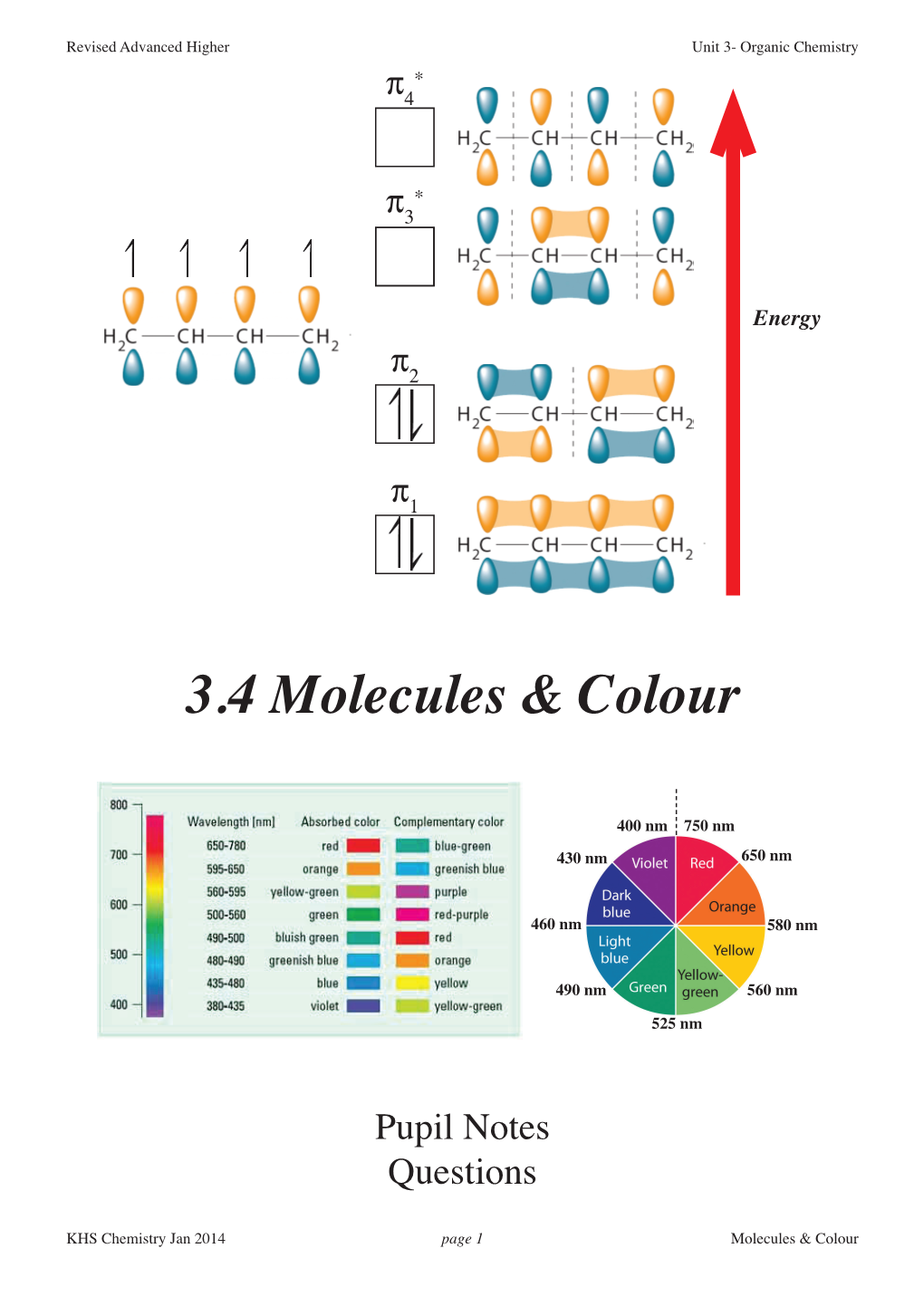
Unit 2- Organic Chemistry Unit 2 - Organic Chemistry
CfE Advanced Higher Unit 2- Organic Chemistry Unit 2 - Organic Chemistry 2.1 Molecular Structure Molecular Orbitals Molecular Structure Colour Stereoisomerism * p4 * p3 Energy p2 p1 400 nm 750 nm 650 nm Pupil Notes 430 nm Learning Outcomes 460 nm 580 nm Questions& Answers 490 nm 560 nm 525 nm KHS Chemistry Nov 2015 page 1 Molecular Structure CfE Advanced Higher Unit 2- Organic Chemistry 2.1 Organic Molecular Structure 2.1.1 Atomic Orbitals and Molecular Orbitals Revisited As early 1923, Louis de Broglie suggested that the properties of electrons in atoms are better explained by treating the electrons as waves rather than as particles. An electron in an atomic orbital is like a stationary, bound vibration: a standing wave, similar to the wave pattern of a guitar string when it is plucked. For a split-second the guitar string is displaced upward. For an instant the wave function, is positive A split-second흍, later the string is displaced downwards. An instant later the wave function, is negative 흍, The wave function, is the mathematical description of the shape of the wave as it vibrates. We are normally more interested in the electron density which is determined by 2 and, therefore, regardless of the sign of the wave흍, function, an identical orbital shape will be produced. 흍 As we've seen before, a plot of electron density for an s-orbital results in a spherical shape and most of the time it has been 'irrelevant' that the original wave function alternates between a positive and a negative value. KHS Chemistry Nov 2015 page 2 Molecular Structure CfE Advanced Higher Unit 2- Organic Chemistry However, when atomic orbitals overlap to form molecular orbitals, the different possible signs on the wave function become more significant and explain whymultiple molecular orbitals will be formed. -

John William Baker and the Origin of the Baker-Nathan Effect
82 Bull. Hist. Chem., VOLUME 37, Number 2 (2012) JOHN WILLIAM BAKER AND THE ORIGIN OF THE BAKER-NATHAN EFFECT Martin D. Saltzman, Providence College, [email protected] The Baker-Nathan effect was a theory proposed by independent school in west London from 1909-1916. He John William Baker (1898-1967) and Wilfred Samuel was awarded a Royal Scholarship in Chemistry which Nathan (1910-1961) of Leeds University in 1935 to allowed him to enroll at the Imperial College of Science explain certain anomalous results obtained in SN2 reac- and Technology in London. His exceptional aptitude for tions carried out in solution. The explanation produced chemistry was shown by his passing the intermediate by them was expanded to a general type of no-bond exams for the B.Sc. Degree by the end of 1916. In April resonance known as hyperconjugation by Mulliken in 1917 he was conscripted and assigned to the Royal En- papers published in 1939 and 1941 (1). Hyperconjuga- gineers where he was commissioned a Lieutenant. His tion is today defined as the conjugation of polarized major responsibilities were in the area of water purifica- sigma bonds and adjacent pi orbitals. This theory has tion. In May 1918 he was sent to Mesopotamia (now Iraq) been applied to a host of anomalous physical measure- where he was assigned the same duties. He was finally ments such as bond lengths and dipole moments. The demobilized in 1920 and returned to Imperial where he Baker-Nathan effect as it was originally framed is no was awarded his B.Sc. -

Downloaded from Bioscientifica.Com at 10/02/2021 11:02:21AM Via Free Access Indicates a Lipophilic Molecule, Whereas a Negative Value Used
Permeability of the mouse zona pellucida: a structure-staining-correlation model using coloured probes K. Turner and R. W. Horobin Department of Biomedicai Science, Western Bank, University of Sheffield, Sheffield SlO 2TN, UK Coloured molecular probes of diverse molecular structure were used to define the permeability of the zona pellucida of preimplantation mouse embryos. The molecular characteristics examined were: electric charge, the size of conjugated aromatic groups and the hydrophilic\p=n-\lipophiliccharacter, all of which can be described numerically. It was hoped that definition of the types of compound able to cross the zona pellucida would contribute to identification and understanding of potential maternal\p=n-\embryonicsignals. The staining pattern of 51 dyes in unfertilized and fertilized one-cell eggs was recorded and 16 dyes were subsequently used to test two-, four- and eight-cell embryos further, to establish whether the permeability of the zona pellucida altered with developmental age. Four different staining patterns were observed that directly correlated with the numerical parameters of the probe but were independent of developmental age. The size of the conjugated system and the hydrophilic\p=n-\lipophilicbalance of the probe were important in determining its interaction with the zona pellucida and embryo whereas electric charge appeared to have little influence. The resulting model is useful for predicting likely interactions between chemically defined molecules and biological entities and suggests that most biologically active molecules and metabolites can pass through the zona pellucida unhindered and enter the embryo with ease. Introduction some bacteria and fungi may also be excluded from the zona pellucida (Eaglesome et al, 1980). -

HYPERCONJUGATION in Conjugation, We Have Studied That the Electrons Move from One P Orbital to Other Which Are Aligned in Parallel Planes
HYPERCONJUGATION In conjugation, we have studied that the electrons move from one p orbital to other which are aligned in parallel planes. Is it possible for electron to jump from p orbital to sp3 orbital that are not parallelly aligned with one another? The answer is yes. This type of conjugation is not normal, it is extra-ordinary. Hence, the name hyper-conjugation. It is also know as no-bond resonance. Let us study more about it. • 3. • Hyperconjugation • The normal electron releasing inductive effect (+I effect) of alkyl groups is in the following order: • But it was observed by Baker and Nathan that in conjugated system, the attachment of alkyl groups reverse their capability of electron releasing. They suggested that alkyl groups are capable of releasing electrons by some process other than inductive. • When a C - H sigma bond is in conjugation, the sigma electrons of this bond enter into conjugation. This is an extension of normal conjugation and was termed hyperconjugation. Unlike the normal conjugation wherein, the electrons get delocalized in p orbitals, here σ electrons move to p orbitals. It involves the delocalisation of C - H sigma electrons. It essentially means that hyper conjugation depends on the presence of alpha-hydrogen atoms. With the increase in number of such hydrogens, the electron releasing effect of alkyl group also increases • The contributing structures involving sigma electrons of C - H bond do not show any covalent bond between C and H. Hyperconjugation, therefore, is also called no bond resonance. • It is important to note that hydrogen does not actually separate from the molecule because if that happens, the necessary condition for resonance to occur will be violated. -

The Simple Hückel Method and Its Applications: Lecture 5
The Simple Hückel Method and its Applications: Lecture 5 Learning outcomes: 1. Understanding cyclic systems, the concept of aromaticity and antiaromaticity 2. Understanding alternant and non-alternant hydrocarbons 7. MOs of Cyclic Conjugated Systems Cyclic conjugated systems are those in which all of the carbons bearing the 2p AOs form a ring. Here we discuss monocyclic conjugated systems in which the conjugated carbon atoms are contained in a single ring, such as cyclopropenyl, cyclobutadiene, benzene, etc. They have higher degrees of symmetry than acyclic systems, and consequently degeneracy in their orbital energies. For example, benzene has a six-fold symmetry axis (D6h), whereas acyclic systems generally have no more than a two-fold (C2) symmetry axis. One result of this is that cyclic conjugated systems typically have a number of degenerate orbitals, i.e. MOs of the same energy but having different spatial distributions of the electrons. Recall that we had learnt that a particle in a square box has a high degree of degeneracy, while the energy levels in a rectangular box are non-degenerate. 7.1 The Cyclopropenyl System The simplest cyclic conjugated system is the three carbon atom cyclopropenyl system According to valence bond theory, it is a resonance hybrid of three structures: 1 Is there a double bond and a radical that swap around in real time? No – we have π- molecular orbitals, which are delocalized over the three atoms. Following the rules for writing the HMO determinant, we obtain: x 1 1 1 x 1 0 1 1 x x(x2 1) 1(x 1) 1(1 x) 0 x3 3x 2 0 (x 1)(x 1)(x 2) 0 x 1,1,2 Note the difference from its acyclic counterpart, the allyl system.