Some Chytrids of Taiwan (II)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Collecting and Recording Fungi
British Mycological Society Recording Network Guidance Notes COLLECTING AND RECORDING FUNGI A revision of the Guide to Recording Fungi previously issued (1994) in the BMS Guides for the Amateur Mycologist series. Edited by Richard Iliffe June 2004 (updated August 2006) © British Mycological Society 2006 Table of contents Foreword 2 Introduction 3 Recording 4 Collecting fungi 4 Access to foray sites and the country code 5 Spore prints 6 Field books 7 Index cards 7 Computers 8 Foray Record Sheets 9 Literature for the identification of fungi 9 Help with identification 9 Drying specimens for a herbarium 10 Taxonomy and nomenclature 12 Recent changes in plant taxonomy 12 Recent changes in fungal taxonomy 13 Orders of fungi 14 Nomenclature 15 Synonymy 16 Morph 16 The spore stages of rust fungi 17 A brief history of fungus recording 19 The BMS Fungal Records Database (BMSFRD) 20 Field definitions 20 Entering records in BMSFRD format 22 Locality 22 Associated organism, substrate and ecosystem 22 Ecosystem descriptors 23 Recommended terms for the substrate field 23 Fungi on dung 24 Examples of database field entries 24 Doubtful identifications 25 MycoRec 25 Recording using other programs 25 Manuscript or typescript records 26 Sending records electronically 26 Saving and back-up 27 Viruses 28 Making data available - Intellectual property rights 28 APPENDICES 1 Other relevant publications 30 2 BMS foray record sheet 31 3 NCC ecosystem codes 32 4 Table of orders of fungi 34 5 Herbaria in UK and Europe 35 6 Help with identification 36 7 Useful contacts 39 8 List of Fungus Recording Groups 40 9 BMS Keys – list of contents 42 10 The BMS website 43 11 Copyright licence form 45 12 Guidelines for field mycologists: the practical interpretation of Section 21 of the Drugs Act 2005 46 1 Foreword In June 2000 the British Mycological Society Recording Network (BMSRN), as it is now known, held its Annual Group Leaders’ Meeting at Littledean, Gloucestershire. -

Chytridiomycetes, Chytridiomycota)
VOLUME 5 JUNE 2020 Fungal Systematics and Evolution PAGES 17–38 doi.org/10.3114/fuse.2020.05.02 Taxonomic revision of the genus Zygorhizidium: Zygorhizidiales and Zygophlyctidales ord. nov. (Chytridiomycetes, Chytridiomycota) K. Seto1,2,3*, S. Van den Wyngaert4, Y. Degawa1, M. Kagami2,3 1Sugadaira Research Station, Mountain Science Center, University of Tsukuba, 1278-294, Sugadaira-Kogen, Ueda, Nagano 386-2204, Japan 2Department of Environmental Science, Faculty of Science, Toho University, 2-2-1, Miyama, Funabashi, Chiba 274-8510, Japan 3Graduate School of Environment and Information Sciences, Yokohama National University, 79-7, Tokiwadai, Hodogaya, Yokohama, Kanagawa 240- 8502, Japan 4Department of Experimental Limnology, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Alte Fischerhuette 2, D-16775 Stechlin, Germany *Corresponding author: [email protected] Key words: Abstract: During the last decade, the classification system of chytrids has dramatically changed based on zoospore Chytridiomycota ultrastructure and molecular phylogeny. In contrast to well-studied saprotrophic chytrids, most parasitic chytrids parasite have thus far been only morphologically described by light microscopy, hence they hold great potential for filling taxonomy some of the existing gaps in the current classification of chytrids. The genus Zygorhizidium is characterized by an zoospore ultrastructure operculate zoosporangium and a resting spore formed as a result of sexual reproduction in which a male thallus Zygophlyctis and female thallus fuse via a conjugation tube. All described species of Zygorhizidium are parasites of algae and Zygorhizidium their taxonomic positions remain to be resolved. Here, we examined morphology, zoospore ultrastructure, host specificity, and molecular phylogeny of seven cultures of Zygorhizidium spp. Based on thallus morphology and host specificity, one culture was identified as Z. -

Systematics of the Lobulomycetales, a New Order Within the Chytridiomycota David Rabern Simmons
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library 5-2007 Systematics of the Lobulomycetales, a New Order within the Chytridiomycota David Rabern Simmons Follow this and additional works at: http://digitalcommons.library.umaine.edu/etd Part of the Botany Commons Recommended Citation Simmons, David Rabern, "Systematics of the Lobulomycetales, a New Order within the Chytridiomycota" (2007). Electronic Theses and Dissertations. 671. http://digitalcommons.library.umaine.edu/etd/671 This Open-Access Dissertation is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. SYSTEMATICS OF THE LOBULOMYCETALES, A NEW ORDER WITHIN THE CHYTRIDIOMYCOTA By David Rabern Simmons B.S. University of Virginia's College at Wise, 2004 A THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science (in Botany and Plant Pathology) The Graduate School University of Maine May, 2007 Advisory Committee: Joyce E. Longcore, Research Associate Professor of Biology, Advisor Seanna L. Annis, Associate Professor of Mycology Seth Tyler, Professor of Zoology and Cooperating Professor of Marine Sciences Copyright 2007 David Rabern Simmons SYSTEMATICS OF THE LOBULOMYCETALES, A NEW ORDER WITHIN THE CHYTRIDIOMYCOTA By David Rabern Simmons Thesis Advisor: Dr. Joyce E. Longcore An Abstract of the Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science (in Botany and Plant Pathology) May, 2007 Based on molecular phylogenetic analyses, the polyphyletic order Chytridiales, one of the four orders in the Chytridiomycota, contains several well- supported clades. -

1993 Li, Heath and Packer.Pdf
The phylogenetic relationships of the anaerobic chytridiomycetous gut fungi (Neocallimasticaceae) and the Chytridiornycota. 11. Cladistic analysis of structural data and description of Neocallimasticales ord.nov. JINLIANCLI, I. BRENTHEATH,] AND LAURENCEPACKER Dep(~rittiet~tof Biology, York Utliversity, North York, Otzt., Cotzodc~M3J IP3 Receivcd May 15, 1992 LI, J., HEATH,I. B., and PACKER,L. 1993. The phylogenetic relationships of the anaerobic chytridiomycetous gut fungi (Neocallimasticaceae) and the Chytridiornycota. 11. Cladistic analysis of structural data and description of Neocalli- masticales ord.nov. Can. J. Bot. 71: 393-407. We investigated the phylogenetic relationships of thc Chytridiomycota and the chytridiomycetous gut fungi with a cladistic analysis of42 morphological, ultrastructural, and mitotic characters for 38 taxa using both maximum parsimony and distance algorithms. Our analyses show that there are three major clades within the Chytridiomycota: the gut fungi, thc Blastocladiales, and the Spizellomycetales-Chytridialcs- Monoblepharidales. Conscqucntly. we elevated the gut fungi to the order Neocallimasticales ord.nov. Our results suggest that a modified Chytridiales, including the Monoblepharidales. is a monophyletic group. In contrast the Spizellomycetales are paraphyletic because the Chytridiales arose within them. The separation of the traditional Chytridiales into two orders is thus doubtful. Although the Blastocladiales are closer to members of the Spizellomycetales than the Chytridiales, the cladistic analyses of both structural and rRNA sequence data do not support the idea that the Blastocladiales were derived from the Spizellomycetales. We suggest emendations to the classification of the Chytridiomycota and note which groupings require further analysis. Our phylogeny for the currently recognized species of gut fungi is inconsis- tent with the existing classification. -

Rhizophlyctidalesda New Order in Chytridiomycota
mycological research 112 (2008) 1031–1048 journal homepage: www.elsevier.com/locate/mycres Rhizophlyctidalesda new order in Chytridiomycota Peter M. LETCHER*, Martha J. POWELL, Donald J. S. BARR, Perry F. CHURCHILL, William S. WAKEFIELD, Kathryn T. PICARD Department of Biological Sciences, The University of Alabama, 411 Hackberry Lane, 319 Biology, Box 870344, Tuscaloosa, AL 35487, USA article info abstract Article history: Rhizophlyctis rosea (Chytridiomycota) is an apparently ubiquitous, soil-inhabiting, cellulose- Received 10 January 2008 degrading chytrid that is the type for Rhizophlyctis. Previous studies have revealed multiple Received in revised form zoospore subtypes among morphologically indistinguishable isolates in the R. rosea com- 27 February 2008 plex sensu Barr. In this study we analysed zoospore ultrastructure and combined nu- Accepted 18 March 2008 rRNA gene sequences (partial LSU and complete ITS1–5.8S–ITS2) of 49 isolates from globally Corresponding Editor: distributed soil samples. Based on molecular monophyly and zoospore ultrastructure, this Gordon W. Beakes group of Rhizophlyctis rosea-like isolates is designated as a new order, the Rhizophlyctidales. Within the Rhizophlyctidales are four new families (Rhizophlyctidaceae, Sonoraphlyctidaceae, Keywords: Arizonaphlyctidaceae, and Borealophlyctidaceae) and three new genera (Sonoraphlyctis, Arizona- Phylogeny phlyctis, and Borealophlyctis). rDNA Rhizophlyctis ª 2008 The British Mycological Society. Published by Elsevier Ltd. All rights reserved. Ultrastructure Zoospore -

Clade (Kingdom Fungi, Phylum Chytridiomycota)
TAXONOMIC STATUS OF GENERA IN THE “NOWAKOWSKIELLA” CLADE (KINGDOM FUNGI, PHYLUM CHYTRIDIOMYCOTA): PHYLOGENETIC ANALYSIS OF MOLECULAR CHARACTERS WITH A REVIEW OF DESCRIBED SPECIES by SHARON ELIZABETH MOZLEY (Under the Direction of David Porter) ABSTRACT Chytrid fungi represent the earliest group of fungi to have emerged within the Kingdom Fungi. Unfortunately despite the importance of chytrids to understanding fungal evolution, the systematics of the group is in disarray and in desperate need of revision. Funding by the NSF PEET program has provided an opportunity to revise the systematics of chytrid fungi with an initial focus on four specific clades in the order Chytridiales. The “Nowakowskiella” clade was chosen as a test group for comparing molecular methods of phylogenetic reconstruction with the more traditional morphological and developmental character system used for classification in determining generic limits for chytrid genera. Portions of the 18S and 28S nrDNA genes were sequenced for isolates identified to genus level based on morphology to seven genera in the “Nowakowskiella” clade: Allochytridium, Catenochytridium, Cladochytrium, Endochytrium, Nephrochytrium, Nowakowskiella, and Septochytrium. Bayesian, parsimony, and maximum likelihood methods of phylogenetic inference were used to produce trees based on one (18S or 28S alone) and two-gene datasets in order to see if there would be a difference depending on which optimality criterion was used and the number of genes included. In addition to the molecular analysis, taxonomic summaries of all seven genera covering all validly published species with a listing of synonyms and questionable species is provided to give a better idea of what has been described and the morphological and developmental characters used to circumscribe each genus. -

Quaeritorhiza Haematococci Is a New Species of Parasitic Chytrid of the Commercially Grown Alga, Haematococcus Pluvialis
Mycologia ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/umyc20 Quaeritorhiza haematococci is a new species of parasitic chytrid of the commercially grown alga, Haematococcus pluvialis Joyce E. Longcore , Shan Qin , D. Rabern Simmons & Timothy Y. James To cite this article: Joyce E. Longcore , Shan Qin , D. Rabern Simmons & Timothy Y. James (2020) Quaeritorhizahaematococci is a new species of parasitic chytrid of the commercially grown alga, Haematococcuspluvialis , Mycologia, 112:3, 606-615, DOI: 10.1080/00275514.2020.1730136 To link to this article: https://doi.org/10.1080/00275514.2020.1730136 View supplementary material Published online: 09 Apr 2020. Submit your article to this journal Article views: 90 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=umyc20 MYCOLOGIA 2020, VOL. 112, NO. 3, 606–615 https://doi.org/10.1080/00275514.2020.1730136 Quaeritorhiza haematococci is a new species of parasitic chytrid of the commercially grown alga, Haematococcus pluvialis Joyce E. Longcore a, Shan Qin b, D. Rabern Simmons c, and Timothy Y. James c aSchool of Biology and Ecology, University of Maine, Orono, Maine 04469-5722; bPhycological LLC, Gilbert, Arizona 85297-1977; cDepartment of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, Michigan 48109-1085 ABSTRACT ARTICLE HISTORY Aquaculture companies grow the green alga Haematococcus pluvialis (Chlorophyta) to extract the Received 28 October 2019 carotenoid astaxanthin to sell, which is used as human and animal dietary supplements. We were Accepted 12 February 2020 requested to identify an unknown pathogen of H. -

The Effect of Metals on Growth, Reproduction and Attachment of Zoosporic True Fungi
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sydney eScholarship The effect of metals on growth, reproduction and attachment of zoosporic true fungi By Linda Henderson School of Life and Environmental Sciences The University of Sydney A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy of The University of Sydney 28 February 2018 Declaration The work described in this thesis is exclusively my own investigation and has not previously been submitted for the award of a degree at this or any other institution. Linda Henderson February 2018 i Authorship attribution statement Chapter 1 of this thesis, excluding Section 1.1, is published as; Henderson, L, Lilje, E. Robinson, K, Gleason FH, Lilje O, 2017. ‘Effects of toxic metals on chytrids, fungal-like organisms and higher fungi’ In: The fungal community: its organisation and role in the ecosystem 4th Ed. J. Dighton and F. White (eds.) CRC Press. The various contributions to this chapter are published within the Contributions section (p. 448) of Henderson et al. 2017. Only those sections of the chapter which I have written (30.1; 30.2.1; 30.3; 30.4; 30.7 in Henderson et al. 2017) have been included in this thesis. Chapter 2 of this thesis is published as; Henderson, L., Ly, M., Robinson, K., Gleason, F., Lilje, O. 2018. Maximum temperature for growth and reproduction is similar in two soil isolates of Gaertneriomyces semiglobifer (Spizellomycetales, Chytridiomycetes) from different soil environments in north eastern New South Wales, Australia. -

Toward a Fully Resolved Fungal Tree of Life
Annual Review of Microbiology Toward a Fully Resolved Fungal Tree of Life Timothy Y. James,1 Jason E. Stajich,2 Chris Todd Hittinger,3 and Antonis Rokas4 1Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, Michigan 48109, USA; email: [email protected] 2Department of Microbiology and Plant Pathology, Institute for Integrative Genome Biology, University of California, Riverside, California 92521, USA; email: [email protected] 3Laboratory of Genetics, DOE Great Lakes Bioenergy Research Center, Wisconsin Energy Institute, Center for Genomic Science and Innovation, J.F. Crow Institute for the Study of Evolution, University of Wisconsin–Madison, Madison, Wisconsin 53726, USA; email: [email protected] 4Department of Biological Sciences, Vanderbilt University, Nashville, Tennessee 37235, USA; email: [email protected] Annu. Rev. Microbiol. 2020. 74:291–313 Keywords First published as a Review in Advance on deep phylogeny, phylogenomic inference, uncultured majority, July 13, 2020 classification, systematics The Annual Review of Microbiology is online at micro.annualreviews.org Abstract https://doi.org/10.1146/annurev-micro-022020- Access provided by Vanderbilt University on 06/28/21. For personal use only. In this review, we discuss the current status and future challenges for fully 051835 Annu. Rev. Microbiol. 2020.74:291-313. Downloaded from www.annualreviews.org elucidating the fungal tree of life. In the last 15 years, advances in genomic Copyright © 2020 by Annual Reviews. technologies have revolutionized fungal systematics, ushering the field into All rights reserved the phylogenomic era. This has made the unthinkable possible, namely ac- cess to the entire genetic record of all known extant taxa. -

High-Level Classification of the Fungi and a Tool for Evolutionary Ecological Analyses
Fungal Diversity (2018) 90:135–159 https://doi.org/10.1007/s13225-018-0401-0 (0123456789().,-volV)(0123456789().,-volV) High-level classification of the Fungi and a tool for evolutionary ecological analyses 1,2,3 4 1,2 3,5 Leho Tedersoo • Santiago Sa´nchez-Ramı´rez • Urmas Ko˜ ljalg • Mohammad Bahram • 6 6,7 8 5 1 Markus Do¨ ring • Dmitry Schigel • Tom May • Martin Ryberg • Kessy Abarenkov Received: 22 February 2018 / Accepted: 1 May 2018 / Published online: 16 May 2018 Ó The Author(s) 2018 Abstract High-throughput sequencing studies generate vast amounts of taxonomic data. Evolutionary ecological hypotheses of the recovered taxa and Species Hypotheses are difficult to test due to problems with alignments and the lack of a phylogenetic backbone. We propose an updated phylum- and class-level fungal classification accounting for monophyly and divergence time so that the main taxonomic ranks are more informative. Based on phylogenies and divergence time estimates, we adopt phylum rank to Aphelidiomycota, Basidiobolomycota, Calcarisporiellomycota, Glomeromycota, Entomoph- thoromycota, Entorrhizomycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota and Olpidiomycota. We accept nine subkingdoms to accommodate these 18 phyla. We consider the kingdom Nucleariae (phyla Nuclearida and Fonticulida) as a sister group to the Fungi. We also introduce a perl script and a newick-formatted classification backbone for assigning Species Hypotheses into a hierarchical taxonomic framework, using this or any other classification system. We provide an example -
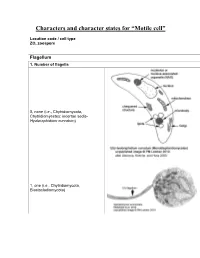
Characters and Character States for “Motile Cell”
Characters and character states for “Motile cell” Location code / cell type ZO, zoospore Flagellum 1. Number of flagella 0, none (i.e., Chytridiomycota, Chytridiomycetes: incertae sedis- Hyaloraphidium curvatum) 1, one (i.e., Chytridiomycota, Blastocladiomycota) 2, multiple (i.e., Neocallimastigomycota) 2. Electron-opaque plug in axoneme core and between axoneme and flagellar membrane 0, absent 1, present (i.e., Chytridiomycota: Chytridiomycetes: Chytridiales, Lobulomycetales, Cladochytriales, incertae sedis: Synchytrium endobioticum, Polychytrium clade; Monoblepharidomycetes) 3. Flagellum coating 0, absent (all taxa except Polyphagus euglenae) 1, present (Polyphagus euglenae) Kinetosome (the term “basal body” is synonymous; Andersen et al. 1991) 4. Electron-opaque core in kinetosome 0, absent (all taxa except Kappamyces) 1, present (Chytridiomycota: Chytridiomycetes: Rhizophydiales- Kappamyces) 5. Scalloped ring within kinetosome, extensions of the A, B, or C microtubule 0, absent 1, present (Lacustromyces hiemalis) Kinetosome-associated structures 6. Kinetosome support 0, absent (Thalassochytrium gracilaripsidis) 1, kinetosome props 2, broken kinetosome props (Olpidium radicale) 3, skirt-like structure surrounding kinetosome (Neocallimastigomycota) 7. Kinetosome-associated plates 0, absent 1, present (Chytridiomycota: Chytridiomycetes: Chytridiales- Group I- type zoospore [Barr 1980]) 8. Kinetosome-associated saddle 0, absent 1, present (Chytridiomycota: Chytridiomycetes: Chytridiales- Group II-type zoospore [Barr 1980]- Chytridium -

Blastocladiomycota) and Rozella Allomycis (Cryptomycota)
fungal biology 121 (2017) 561e572 journal homepage: www.elsevier.com/locate/funbio Ultrastructural characterization of the hosteparasite interface between Allomyces anomalus (Blastocladiomycota) and Rozella allomycis (Cryptomycota) Martha J. POWELLa, Peter M. LETCHERa,*, Timothy Y. JAMESb aDepartment of Biological Sciences, The University of Alabama, Tuscaloosa, AL 35487, USA bDepartment of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA article info abstract Article history: Rozella allomycis is an obligate endoparasite of the water mold Allomyces and a member of Received 16 December 2016 a clade (¼ Opisthosporidia) sister to the traditional Fungi. Gaining insights into Rozella’s de- Received in revised form velopment as a phylogenetically pivotal endoparasite can aid our understanding of struc- 8 March 2017 tural adaptations and evolution of the Opisthosporidia clade, especially within the context Accepted 13 March 2017 of genomic information. The purpose of this study is to characterize the interface between Available online 21 March 2017 R. allomycis and Allomyces anomalus. Electron microscopy of developing plasmodia of R. al- Corresponding Editor: lomycis in host hyphae shows that the interface consists of three-membrane layers, inter- Gordon William Beakes preted as the parasite’s plasma membrane (inner one layer) and a host cisterna (outer two layers). As sporangial and resting spore plasmodia develop, host mitochondria typically Keywords: cluster at the surface of the parasite and eventually align parallel to the three-membrane Evolution layered interface. The parasite’s mitochondria have only a few cristae and the mitochon- Interface drial matrix is sparse, clearly distinguishing parasite mitochondria from those of the Mitochondrial recruitment host. Consistent with the expected organellar topology if the parasite plasmodia phagocy- Parasitism tize host cytoplasm, phagocytic vacuoles are at first bounded by three-membrane layers Phagocytosis with host-type mitochondria lining the inner membrane.