Methionine Production – a Critical Review
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Effects of Single Amino Acid Deficiency on Mrna Translation Are Markedly
www.nature.com/scientificreports OPEN Efects of single amino acid defciency on mRNA translation are markedly diferent for methionine Received: 12 December 2016 Accepted: 4 May 2018 versus leucine Published: xx xx xxxx Kevin M. Mazor, Leiming Dong, Yuanhui Mao, Robert V. Swanda, Shu-Bing Qian & Martha H. Stipanuk Although amino acids are known regulators of translation, the unique contributions of specifc amino acids are not well understood. We compared efects of culturing HEK293T cells in medium lacking either leucine, methionine, histidine, or arginine on eIF2 and 4EBP1 phosphorylation and measures of mRNA translation. Methionine starvation caused the most drastic decrease in translation as assessed by polysome formation, ribosome profling, and a measure of protein synthesis (puromycin-labeled polypeptides) but had no signifcant efect on eIF2 phosphorylation, 4EBP1 hyperphosphorylation or 4EBP1 binding to eIF4E. Leucine starvation suppressed polysome formation and was the only tested condition that caused a signifcant decrease in 4EBP1 phosphorylation or increase in 4EBP1 binding to eIF4E, but efects of leucine starvation were not replicated by overexpressing nonphosphorylatable 4EBP1. This suggests the binding of 4EBP1 to eIF4E may not by itself explain the suppression of mRNA translation under conditions of leucine starvation. Ribosome profling suggested that leucine deprivation may primarily inhibit ribosome loading, whereas methionine deprivation may primarily impair start site recognition. These data underscore our lack of a full -

Enzymatic Assay of L-METHIONINE GAMMA-LYASE (EC 4.4.1.11)
Enzymatic Assay of L-METHIONINE GAMMA-LYASE (EC 4.4.1.11) PRINCIPLE: L-Methionine Gamma-Lyase L-Methionine > Methanethiol + 2-Ketobutyrate + NH3 2-Ketobutyrate + MBTH > Azine Derivative Abbreviation used: MBTH = 3-Methyl-2-Benzothiazolinone CONDITIONS: T = 37°C, pH = 8.0, A320nm, Light path = 1 cm METHOD: Stopped Spectrophotometric Rate Determination REAGENTS: A. 100 mM Potassium Phosphate Buffer with 25 mM L-Methionine and 0.01 mM Pyridoxal 5-Phosphate, pH 8.0 at 37°C1 (Prepare 100 ml in deionized water using Potassium Phosphate, Monobasic, Anhydrous, Sigma Prod. No. P-5379, L-Methionine, Sigma Prod. No. M-9625, and Pyridoxal 5-Phosphate, Sigma Prod. No. P-9255. Adjust to pH 8.0 at 37°C with 5 M KOH.) B. 50% (w/v) Trichloroacetic Acid Solution (TCA) (Prepare 5 ml in deionized water using Trichloroacetic Acid, 6.1 N Solution, approximately 100% (w/v), Sigma Stock No. 490-10.) C. 1 M Sodium Acetate Buffer, pH 5.0 at 37°C (NaOAC) (Prepare 100 ml in deionized water using Sodium Acetate, Trihydrate, Sigma Prod. No. S-8625. Adjust to pH 5.0 at 37°C with 5 M HCl.) D. 0.1% (w/v) 3-Methyl-2-Benzothiazolinone Hydrazone (MBTH) (Prepare 10 ml in deionized water using 3-Methyl-2-Benzothiazolinone Hydrazone, Hydrochloride Hydrate, Sigma Prod. No. M-8006.) SSMETH01 Page 1 of 4 07/29/98 Enzymatic Assay of L-METHIONINE GAMMA-LYASE (EC 4.4.1.11) REAGENTS: E. 100 mM Potassium Phosphate Buffer with 1 mM Ethylenediaminetetraacetic Acid, 0.01% (v/v) 2-Mercaptoethanol and 0.02 mM Pyridoxal 5-Phosphate, pH 7.2 at 37°C (Enzyme Diluent)1 (Prepare 10 ml in deionized water using Potassium Phosphate, Monobasic, Anhydrous, Sigma Prod. -

Relative Reaction Rates of the Amino Acids Cysteine, Methionine, and Histidine with Analogs of the Anti-Cancer Drug Cisplatin Cynthia A
Western Kentucky University TopSCHOLAR® Honors College Capstone Experience/Thesis Honors College at WKU Projects 5-11-2015 Relative Reaction Rates of the Amino Acids Cysteine, Methionine, and Histidine with Analogs of the Anti-Cancer Drug Cisplatin Cynthia A. Tope Western Kentucky University, [email protected] Follow this and additional works at: http://digitalcommons.wku.edu/stu_hon_theses Part of the Medicinal-Pharmaceutical Chemistry Commons Recommended Citation Tope, Cynthia A., "Relative Reaction Rates of the Amino Acids Cysteine, Methionine, and Histidine with Analogs of the Anti-Cancer Drug Cisplatin" (2015). Honors College Capstone Experience/Thesis Projects. Paper 571. http://digitalcommons.wku.edu/stu_hon_theses/571 This Thesis is brought to you for free and open access by TopSCHOLAR®. It has been accepted for inclusion in Honors College Capstone Experience/ Thesis Projects by an authorized administrator of TopSCHOLAR®. For more information, please contact [email protected]. RELATIVE REACTION RATES OF THE AMINO ACIDS CYSTEINE, METHIONINE, AND HISTIDINE WITH ANALOGS OF THE ANTI-CANCER DRUG CISPLATIN A Capstone Experience/Thesis Project Presented in Partial Fulfillment of the Requirements for the Degree Bachelor of Science with Honors College Graduate Distinction at Western Kentucky University By: Cynthia A. Tope ***** Western Kentucky University 2015 CE/T Committee: Approved by: Professor Kevin Williams, Advisor _________________________ Professor Darwin Dahl Advisor Professor Lee Ann Smith Department of Chemistry Copyright: Cynthia A. Tope 2015 ABSTRACT We are studying the reaction of analogs of the anticancer drug cisplatin with amino acids that differ in size and shape. The reaction of cisplatin with proteins likely precedes reaction with DNA in the body, forming a variety of products that may be toxic to the human body. -
![Lyxumia Subcutaneous Injection 300 Μg [Non-Proprietary Name] Lixisenatide (JAN*) [Name of Applicant] Sanofi K.K](https://docslib.b-cdn.net/cover/4198/lyxumia-subcutaneous-injection-300-g-non-proprietary-name-lixisenatide-jan-name-of-applicant-sanofi-k-k-324198.webp)
Lyxumia Subcutaneous Injection 300 Μg [Non-Proprietary Name] Lixisenatide (JAN*) [Name of Applicant] Sanofi K.K
Report on the Deliberation Results May 31, 2013 Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry of Health, Labour and Welfare [Brand name] Lyxumia Subcutaneous Injection 300 μg [Non-proprietary name] Lixisenatide (JAN*) [Name of applicant] Sanofi K.K. [Date of application] June 11, 2012 [Results of deliberation] In the meeting held on May 24, 2013, the First Committee on New Drugs concluded that the product may be approved and that this result should be reported to the Pharmaceutical Affairs Department of the Pharmaceutical Affairs and Food Sanitation Council. The re-examination period for the product is 8 years, and the drug substance and the drug product are both classified as powerful drugs and the product is not classified as a biological product or a specified biological product. The proposed Japanese brand name should be changed for ensuring medical safety. *Japanese Accepted Name (modified INN) This English version of the Japanese review report is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. The PMDA will not be responsible for any consequence resulting from the use of this English version. Review Report May 7, 2013 Pharmaceuticals and Medical Devices Agency The results of a regulatory review conducted by the Pharmaceuticals and Medical Devices Agency on the following pharmaceutical product submitted for registration are as follows. [Brand name] Lyxumia Subcutaneous -

The Un-Design and Design of Insulin: Structural Evolution
THE UN-DESIGN AND DESIGN OF INSULIN: STRUCTURAL EVOLUTION WITH APPLICATION TO THERAPEUTIC DESIGN By NISCHAY K. REGE Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Biochemistry Dissertation Advisor: Dr. Michael A. Weiss CASE WESTERN RESERVE UNIVERSITY August, 2018 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Nischay K. Rege candidate for the degree of Doctor of Philosophy*. Committee Chair Paul Carey Committee Member Michael Weiss Committee Member Faramarz Ismail-Beigi Committee Member George Dubyak Date of Defense: June 26th, 2018 *We also certify that written approval has been obtained for any proprietary material contained therein. Dedication This thesis is dedicated to my mother, Dipti, whose constant love and faith have never failed, to my father, Kiran, who taught me of the virtue of curiosity, and to my wife, Shipra, whose kindness and companionship have given me enough strength for eight lifetimes. i Table of Contents Dedication ..................................................................................................................................... i Table of Contents ......................................................................................................................... ii List of Tables ............................................................................................................................... v List of Figures ........................................................................................................................... -

Genetic Studies of Leptin Concentrations Implicate Leptin in the Regulation of Early
Page 1 of 93 Diabetes Genetic studies of leptin concentrations implicate leptin in the regulation of early adiposity Hanieh Yaghootkar1,2,3*&, Yiying Zhang4&, Cassandra N Spracklen5, Tugce Karaderi6,7,8,9, Lam Opal Huang10, Jonathan Bradfield11,12, Claudia Schurmann13, Rebecca S Fine14,15,16, Michael H Preuss13, Zoltan Kutalik1,17,18, Laura BL Wittemans6,19, Yingchang Lu13,20, Sophia Metz10, Sara M Willems19, Ruifang Li-Gao21, Niels Grarup10, Shuai Wang22, Sophie Molnos23,24, América A Sandoval-Zárate25, Mike A Nalls26,27, Leslie A Lange28, Jeffrey Haesser29, Xiuqing Guo30, Leo-Pekka Lyytikäinen31,32, Mary F Feitosa33, Colleen M Sitlani34, Cristina Venturini35, Anubha Mahajan6,36, Tim Kacprowski37,38, Carol A Wang22, Daniel I Chasman39,40, Najaf Amin41, Linda Broer42, Neil Robertson6,36, Kristin L Young43, Matthew Allison44, Paul L Auer45, Matthias Blüher46, Judith B Borja47,48, Jette Bork-Jensen10, Germán D Carrasquilla10, Paraskevi Christofidou35, Ayse Demirkan41, Claudia A Doege49, Melissa E Garcia50, Mariaelisa Graff43,51, Kaiying Guo4, Hakon Hakonarson11,52, Jaeyoung Hong22, Yii-Der Ida Chen30, Rebecca Jackson53, Hermina Jakupović10, Pekka Jousilahti54, Anne E Justice55, Mika Kähönen56,57, Jorge R Kizer58,59, Jennifer Kriebel23,24, Charles A LeDuc4, Jin Li60, Lars Lind61, Jian’an Luan19, David Mackey62, Massimo Mangino35,63, Satu Männistö54, Jayne F Martin Carli4, Carolina Medina-Gomez41,42, Dennis O Mook-Kanamori21,64, Andrew P Morris65,6,66, Renée de Mutsert21, Matthias Nauck67,38, Ivana Nedeljkovic41, Craig E Pennell22, Arund D Pradhan39,40, Bruce M Psaty68,69, Olli T Raitakari70,71,72, Robert A Scott19, Tea Skaaby73, Konstantin Strauch74,75, Kent D Taylor30, Alexander Teumer76,38, Andre G Uitterlinden41,42, Ying Wu5, Jie Yao30, Mark Walker77, Kari E North43, Peter Kovacs46, M. -

L-Methionine
October 24, 2011 Lisa Brines, Ph.D. National List Manager USDA/AMS/NOP, Standards Division 1400 Independence Ave. SW Room 2646-So., Ag Stop 0268 Washington, DC 20250-0268 RE: Petition for inclusion of L-Methionine on the National List at §205.605(b) as a synthetic non-agricultural substance allowed in or on processed infant formula products labeled as “organic” or “made with organic (specified ingredients)” with the annotation “for use only in infant formula based on isolated soy protein.” Dear Dr. Brines, The International Formula Council (IFC) is an association of manufacturers and marketers of formulated nutrition products (e.g., infant formulas and adult nutritionals) whose members are based predominantly in North America. IFC members support the American Academy of Pediatrics’ (AAP) position that breastfeeding is the preferred method of feeding infants. We also agree with the AAP that, for infants who do not receive breast milk, iron-fortified infant formula is the only safe and recommended alternative, IFC members are committed to providing infant formulas of the highest quality for those mothers who cannot or choose not to breastfeed, discontinue breastfeeding prior to one year or choose to supplement. This petition seeks to add L-Methionine to the National List to permit its addition as a nonagricultural ingredient in infant formula based on isolated soy protein. L-Methionine is an essential amino acid for humans of all ages. Amino acids are the building blocks of protein. An essential amino acid is one that must be provided in the foods in our diet since our bodies do not have the capability of producing enough of it for normal metabolism and growth. -

Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat
Pediat. Res. 6: 713-719 (1972) Amino acid neonate intestine transport, amino acid Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat J. F. FITZGERALD1431, S. REISER, AND P. A. CHRISTIANSEN Departments of Pediatrics, Medicine, and Biochemistry, and Gastrointestinal Research Laboratory, Indiana University School of Medicine and Veterans Administration Hospital, Indianapolis, Indiana, USA Extract The activity of amino acid transport pathways in the small intestine of the 2-day-old rat was investigated. Transport was determined by measuring the uptake of 1 mM con- centrations of various amino acids by intestinal segments after a 5- or 10-min incuba- tion and it was expressed as intracellular accumulation. The neutral amino acid transport pathway was well developed with intracellular accumulation values for leucine, isoleucine, valine, methionine, tryptophan, phenyl- alanine, tyrosine, and alanine ranging from 3.9-5.6 mM/5 min. The intracellular accumulation of the hydroxy-containing neutral amino acids threonine (essential) and serine (nonessential) were 2.7 mM/5 min, a value significantly lower than those of the other neutral amino acids. The accumulation of histidine was also well below the level for the other neutral amino acids (1.9 mM/5 min). The basic amino acid transport pathway was also operational with accumulation values for lysine, arginine and ornithine ranging from 1.7-2.0 mM/5 min. Accumulation of the essential amino acid lysine was not statistically different from that of nonessential ornithine. Ac- cumulation of aspartic and glutamic acid was only 0.24-0.28 mM/5 min indicating a very low activity of the acidic amino acid transport pathway. -
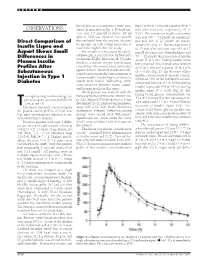
OBSERVATIONS Sured, As Indicated in Fig
LETTERS blood glucose concentrations were mea- lispro at 40 Ϯ 3 min and aspart at 49 Ϯ 3 OBSERVATIONS sured, as indicated in Fig. 1. If blood glu- min after injection, respectively (P ϭ cose was 3.5 mmol/l or lower, 20 ml 0.01). The maximum insulin concentra- glucose 30% was injected. One patient tion was 316 Ϯ 31 pmol/l on insulin lis- Direct Comparison of was excluded from the analysis because, pro and 295 Ϯ 27 pmol/l on insulin by mistake, he took a large extra dose of aspart (NS) (Fig. 1). The increase from 0 Insulin Lispro and insulin the night before the study. to 15 min after injection was 109 Ϯ 17 Aspart Shows Small Free insulin was measured after poly- pmol/l after injection of insulin lispro and Differences in ethylene glycol precipitation by Mercodia 53 Ϯ 11 pmol/l after injection of insulin Iso-Insulin (ELISA; Mercodia AB, Uppsala, Plasma Insulin aspart (P ϭ 0.02). Fasting insulin lispro Sweden), a two-site enzyme immunoassay levels reached 50% of peak concentration Profiles After containing two monoclonal antibodies at 20 Ϯ 1 min and aspart at 30 Ϯ 3 min against insulin. Identical results were ob- (P ϭ 0.02) (Fig. 2). The decrease of free Subcutaneous tained when equimolar concentrations of Injection in Type 1 insulin concentration from peak concen- human insulin, insulin lispro, and insulin tration to 50% of the maximum concen- aspart were tested, indicating 100% Diabetes tration was found at 113 Ϯ 10 min during cross-reactivity between lispro, aspart, insulin lispro and 154 Ϯ 14 min during and human insulin in this assay. -

Amino Acid Degradation
BI/CH 422/622 OUTLINE: OUTLINE: Protein Degradation (Catabolism) Digestion Amino-Acid Degradation Inside of cells Protein turnover Dealing with the carbon Ubiquitin Fates of the 29 Activation-E1 Seven Families Conjugation-E2 nitrogen atoms in 20 1. ADENQ Ligation-E3 AA: Proteosome 2. RPH 9 ammonia oxidase Amino-Acid Degradation 18 transamination Ammonia 2 urea one-carbon metabolism free transamination-mechanism to know THF Urea Cycle – dealing with the nitrogen SAM 5 Steps Carbamoyl-phosphate synthetase 3. GSC Ornithine transcarbamylase PLP uses Arginino-succinate synthetase Arginino-succinase 4. MT – one carbon metabolism Arginase 5. FY – oxidase vs oxygenase Energetics Urea Bi-cycle 6. KW – Urea Cycle – dealing with the nitrogen 7. BCAA – VIL Feeding the Urea Cycle Glucose-Alanine Cycle Convergence with Fatty acid-odd chain Free Ammonia Overview Glutamine Glutamate dehydrogenase Overall energetics Amino Acid A. Concepts 1. ConvergentDegradation 2. ketogenic/glucogenic 3. Reactions seen before The SEVEN (7) Families B. Transaminase (A,D,E) / Deaminase (Q,N) Family C. Related to biosynthesis (R,P,H; C,G,S; M,T) 1.Glu Family a. Introduce oxidases/oxygenases b. Introduce one-carbon metabolism (1C) 2.Pyruvate Family a. PLP reactions 3. a-Ketobutyric Family (M,T) a. 1-C metabolism D. Dedicated 1. Aromatic Family (F,Y) a. oxidases/oxygenases 2. a-Ketoadipic Family (K,W) 3. Branched-chain Family (V,I,L) E. Convergence with Fatty Acids: propionyl-CoA 29 N 1 Amino Acid Degradation • Intermediates of the central metabolic pathway • Some amino acids result in more than one intermediate. • Ketogenic amino acids can be converted to ketone bodies. -

Where Metal Ions Bind in Proteins (Metafloprotein/Protein Structure/Hydrophobicity Contrast Function) MASON M
Proc. Nadl. Acad. Sci. USA Vol. 87, pp. 5648-5652, August 1990 Biophysics Where metal ions bind in proteins (metafloprotein/protein structure/hydrophobicity contrast function) MASON M. YAMASHITA*t, LAURA WESSON*, GEORGE EISENMANt, AND DAVID EISENBERG*§ *Molecular Biology Institute and Department of Chemistry and Biochemistry, and *Department of Physiology, University of California, Los Angeles, CA 90024 Contributed by David Eisenberg, May 14, 1990 ABSTRACT The environments of metal ions (Li', Na', K+, Ag+, Cs+, Mg2+, Ca2+, Mn2+9 Cu2+, Zn2+) in proteins and other metal-host molecules have been examined. Regard- .. 0.00- ______ less ofthe metal and its precise pattern ofligation to the protein, there is a common qualitative feature to the bind site: the metal is ligated by a shell of hydrophilic atomic groups (con- E -0.01 Zn2+ taining oxygen, nitrogen, or sulfur atoms) and this hydrophilic shell is embedded within a larger shell of hydrophobic atomic - -0.02 groups (containing carbon atoms). That is, metals bind at centers of high hydrophobicity contrast. This qualitative ob- servation can be described analytically by the hydrophobicity 0 2 4 6 8 10 contrast function, C, evaluated from the structure. This func- tion is large and positive for a sphere of hydrophilic atomic 0.01 groups (characterized by atomic salvation parameters, Aar, having values < 0) at the center of a larger sphere of hydro- phobic atomic groups (characterized by Aor > 0). In the 23 0.00. metal-binding molecules we have examined, the maximum values of the contrast function lie near to observed metal binding sites. This suggests that the hydrophobicity contrast function may be useful for locating, characterizing, and de- - -0.01 signing metal binding sites in proteins. -

Thionein Can Serve As a Reducing Agent for the Methionine Sulfoxide Reductases
Thionein can serve as a reducing agent for the methionine sulfoxide reductases Daphna Sagher*†, David Brunell*†, J. Fielding Hejtmancik‡, Marc Kantorow*, Nathan Brot§, and Herbert Weissbach*¶ *Center for Molecular Biology and Biotechnology, Florida Atlantic University, Boca Raton, FL 33431; ‡Ophthalmic Genetic and Visual Function Branch, National Eye Institute, National Institutes of Health, Bethesda, MD 20892; and §Department of Microbiology and Immunology, Hospital for Special Surgery, Cornell University Medical Center, New York, NY 10021 Contributed by Herbert Weissbach, April 7, 2006 It has been generally accepted, primarily from studies on methio- not been reported. Most in vitro studies have used DTT as the nine sulfoxide reductase (Msr) A, that the biological reducing agent reducing agent for both MsrA and MsrB, because this agent for the members of the Msr family is reduced thioredoxin (Trx), works very well with the Msr family of proteins. although high levels of DTT can be used as the reductant in vitro. Recently, the human MsrB genes have been of interest to us as Preliminary experiments using both human recombinant MsrB2 part of our studies on the role of the Msr system in protecting lens (hMsrB2) and MsrB3 (hMsrB3) showed that although DTT can and retinal cells against oxidative damage (18–20). Using a color- function in vitro as the reducing agent, Trx works very poorly, imetric assay for Msr activity, based on the reduction of the prompting a more careful comparison of the ability of DTT and Trx individual epimers of 4-N,N-dimethylaminoazobenzene-4-sulfonyl to function as reducing agents with the various members of the (DABS)-Met(o), we were surprised to find that Trx serves very Msr family.