No Evidence for a Magnetite-Based Magnetoreceptor in the Lagena Of
Total Page:16
File Type:pdf, Size:1020Kb
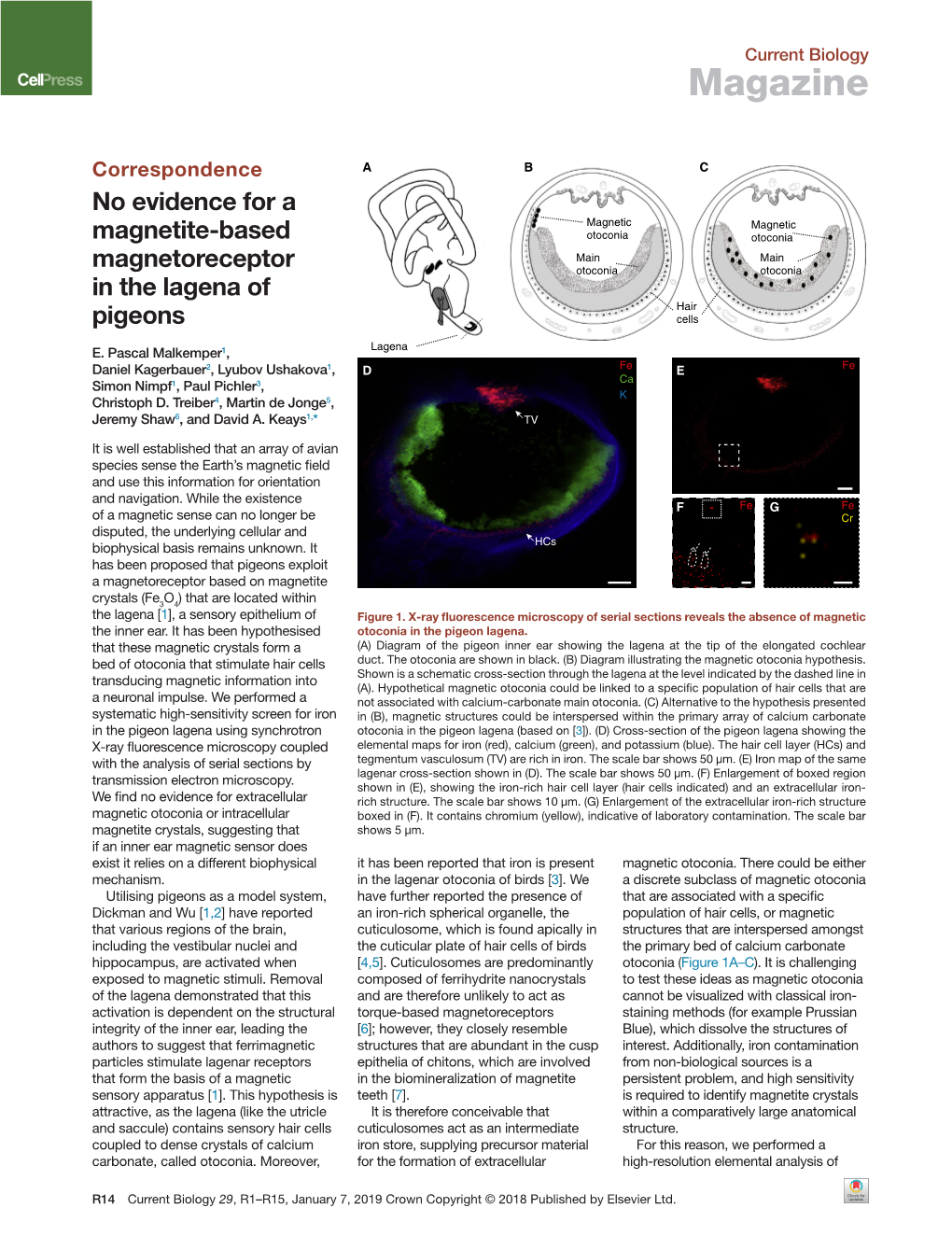
Load more
Recommended publications
-

Independence of the Endovestibular Potential in Homeotherms
Independence of the Endovestibular Potential in Homeotherms ROBERT S. SCHMIDT From the Department of Surgery (Otolaryngology), University of Chicago, Chicago ABS TR Ac T The endolymphatic potential was recorded from various vestibular parts of the labyrinth from which the cochlea (in the case of guinea pigs) or the cochlea, lagena, and sacculus (in the case of pigeons) had been removed. This endovesfibular potential of the isolated vestibule declined during anoxia and recovered after anoxia in the same manner as the endovestibular potential of the intact labyrinth. Its non-anoxic level was the same as in the intact laby- rinth; i.e., +5 to -[-8 mv in the pigeon and +2 to +5 mv in the guinea pig. It is, therefore, concluded that the endovestibular potential is independent of the cochlea, stria vascularis, and endocochlear potential. INTRODUCTION The endolymphatic potential discovered by B~k~sy (1) has been studied in both the cochlea (2) and vestibule (3, 4). This potential in the cochlea, the endocochlear potential (ECP), is about 80 mv positive in mammals and about 15 mv positive in birds (5). The potential in the vestibular parts of the labyrinth, the endovestibular potential (EVP), is much lower in homeotherms (4--6). Three assumptions regarding the EVP are quite common (4, 7, 8):--that nothing compared to the stria vascularis, the probable source of the ECP, is found in the vestibule; that the EVP results merely from spread of the ECP; and that the EVP is therefore of little interest or importance. These assumptions have very little theoretical or experimental foundation. -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

Identification of a 275-Kd Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G Richardson, Sylvain Bartolami, I Russell
Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G Richardson, Sylvain Bartolami, I Russell To cite this version: G Richardson, Sylvain Bartolami, I Russell. Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear. Journal of Cell Biology, Rockefeller University Press, 1990, 110, 10.1083/jcb.110.4.1055. hal-02156410 HAL Id: hal-02156410 https://hal.archives-ouvertes.fr/hal-02156410 Submitted on 18 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G. P. Richardson, S. Bartolami, and I. J. Russell School of Biological Sciences, University of Sussex, Falmer, Brighton, United Kingdom Abstract. Immunological techniques have been used basilar papilla (an auditory organ) only the proximal to generate both polyclonal and monoclonal antibodies region of the stereocilia bundle nearest to the apical specific for the apical ends of sensory hair cells in the surface is stained. The monoclonal anti-hair cell anti- avian inner ear. -

The Development of the Inner Ear of the Lizard (Sceloporus Undulatus)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Illinois Digital Environment for Access to Learning and Scholarship Repository Digitized by the Internet Archive in 2013 http://archive.org/details/developmentofinnOOgour THE DEVELOPMENT OF THE INNER EAR OF THE LIZARD, (SCELOPORUS UNDULATUS) BY MARY JANE GOURLEY A. B. University of Illinois, 1909. THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF ARTS IN ZOOLOGY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS^ 1914 UNIVERSITY OF ILLINOIS THE GRADUATE SCHOOL 190 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE Recommendation concurred in: Committee i on Final Examination II 284573 UlUC TABLE OF CONTENTS Page I Introduction _______ i Methods and Materials II Development of the Inner Ear of the Lizard Stage I _______ 1 Stage II - __-__ - 2 Stage III _______ 3 Stage IV------ - 4 Stage V _______ 5 Stage VI------ - 7 Stage VII------ - 8 Stage VIII _______ 8 III SuMfiary ________ 9 Literature 10 _______ Explanation of Plates - • - - - - 12 Plates ________ 13 . 1 I INTRODUCTION The following paper describes the embryonic development of the inner ear and especially the membranous labyrinth of the lizard ( Soel oporus undulatus ) . Saggital and transverse serial sections of various stages in the development of these animals were studied and wax reconstructions were made of the important stages. Then the sensory area of each section was outlined and painted on the model, so that they shov/ed the limits of the sensory and non-sensory epi- thelium. -

Ectopic Otoconial Formation in the Lagena of the Pigeon Inner Ear E
© 2018. Published by The Company of Biologists Ltd | Biology Open (2018) 7, bio034462. doi:10.1242/bio.034462 RESEARCH ARTICLE Ectopic otoconial formation in the lagena of the pigeon inner ear E. Pascal Malkemper1,*, Matthew J. Mason2, Daniel Kagerbauer3, Simon Nimpf1 and David A. Keays1,* ABSTRACT to low-frequency seismic vibrations (Lewis et al., 1982). In birds and The vertebrate inner ear contains vestibular receptors with dense reptiles, the lagena represents a separate endorgan at the apical tip of crystals of calcium carbonate, the otoconia. The production and the auditory basilar papilla (Smith and Takasaka, 1971). The structural maintenanceofotoconiaisadelicateprocess,theperturbationofwhich similarity to the vestibular utricle and saccule, the absence of responses can lead to severe vestibular dysfunction in humans. The details of to auditory stimuli, and the exclusive innervation of vestibular these processes are not well understood. Here, we report the discovery brainstem nuclei render a vestibular function of the avian lagena likely of a new otoconial mass in the lagena of adult pigeons that was present (Kaiser and Manley, 1996; Manley et al., 1991). The striola of the in more than 70% of birds. Based on histological, tomographic and lagena is aligned at an angle of 31-45° to the perpendicularly-aligned elemental analyses, we conclude that the structure likely represents utricle and saccule and thus would collect useful additional information an ectopically-formed otoconial assembly. Given its frequent natural in three-dimensional space (Ishiyama, 1995). Additionally, synchrotron occurrence, we suggest that the pigeon lagena is a valuable model x-ray fluorescence (XRF) measurements have revealed iron in the system for investigating misregulated otoconial formation. -

An Access-Dictionary of Internationalist High Tech Latinate English
An Access-Dictionary of Internationalist High Tech Latinate English Excerpted from Word Power, Public Speaking Confidence, and Dictionary-Based Learning, Copyright © 2007 by Robert Oliphant, columnist, Education News Author of The Latin-Old English Glossary in British Museum MS 3376 (Mouton, 1966) and A Piano for Mrs. Cimino (Prentice Hall, 1980) INTRODUCTION Strictly speaking, this is simply a list of technical terms: 30,680 of them presented in an alphabetical sequence of 52 professional subject fields ranging from Aeronautics to Zoology. Practically considered, though, every item on the list can be quickly accessed in the Random House Webster’s Unabridged Dictionary (RHU), updated second edition of 2007, or in its CD – ROM WordGenius® version. So what’s here is actually an in-depth learning tool for mastering the basic vocabularies of what today can fairly be called American-Pronunciation Internationalist High Tech Latinate English. Dictionary authority. This list, by virtue of its dictionary link, has far more authority than a conventional professional-subject glossary, even the one offered online by the University of Maryland Medical Center. American dictionaries, after all, have always assigned their technical terms to professional experts in specific fields, identified those experts in print, and in effect held them responsible for the accuracy and comprehensiveness of each entry. Even more important, the entries themselves offer learners a complete sketch of each target word (headword). Memorization. For professionals, memorization is a basic career requirement. Any physician will tell you how much of it is called for in medical school and how hard it is, thanks to thousands of strange, exotic shapes like <myocardium> that have to be taken apart in the mind and reassembled like pieces of an unpronounceable jigsaw puzzle. -

Mechanoreception
Mechanoreception Introduction Hair cells : the basic mechanosensory unit Hair cell structure Inner ear and accessory organ structures Vestibule Otolith organs Weberian ossicles Lateral line Lateral line structure Receptor organs Acoustic communication: sound production and reception Sound production mechanisms Locomotion and posture Introduction A mechanoreceptor is a sensory receptor that responds to mechanical pressure or distortion. In fishes mechanoreception concerns the inner ear and the lateral line system. Hair cells are the UNIVERSAL MECHANOSENSORY TRANSDUCERS in both the lateral line and hearing systems. The INNER EAR is responsible for fish EQUILIBRIUM, BALANCE and HEARING LATERAL LINE SYSTEM detects DISTURBANCES in the water. Hair cell structure EACH HAIR CELL CONSISTS OF TWO TYPES OF "HAIRS" OR RECEPTOR PROCESSES: Many microvillar processes called STEREOCILIA. One true cilium called the KINOCILLIUM. COLLECTIVELY, the cluster is called a CILIARY BUNDLE. The NUMBER OF STEREOCILIA PER BUNDLE IS VARIABLE, and ranges from a 10s of stereocilia to more than a 100. The STEREOCILIA PROJECT into a GELATINOUS CUPULA ON THE APICAL (exposed) SURFACE of the cell. The cilium and villi are ARRANGED IN A STEPWISE GRADATION - the longest hair is the kinocillium, and next to it, the stereocilia are arranged in order of decreasing length. These cells SYNAPSE WITH GANGLION CELLS. They have DIRECTIONAL PROPERTIES - response to a stimulus depends on the direction in which the hairs are bent. So, if the displacement causes the stereocilia to bend towards the kinocilium, the cell becomes DEPOLARIZED = EXCITATION. If the stereocilia bend in the opposite direction, the cell becomes HYPERPOLARIZED = INHIBITION of the cell. If the hair bundles are bent at a 90o angle to the axis of the kinocilium and stereocilia there will be no response. -

Thieme: Veterinary Anatomy of Domestic Animals
Index Page numbers in bold indicate references in figures. A Abducent nerve (VI) 521, 524, 528, Acetabulum 122, 243, 246, 247, Alar 550–551, 554, 560, 611, 615 247–250, 749 – canal 79 Abaxial 26 Abductor muscle/s 47 Acetylcholinase 65 – cartilages 398,402 – – 202 collateral ligament 200 201, , – of thigh 288, 292 Acetylcholine 44, 64–65, 581 –– ossification 730 204 Ablatio retinae 697 Achilles tendon 296–297, 302 – fold 402 – digital Abnormal behaviour 699 Acinous glands 67 – foramen 110, 115, 129–130, 626 –– 667 artery 665, Abomasal velum 361 Acoustic – ligament 130 –– 668 nerves Abomasopexy 715 – area 536 , 556 – notch 110 –– 667 veins Abomasum 360, 362, 366, 367, 378, – organs 524 Albino animals 697 – dorsal proper artery of fourth 720–721 – radiation 536 Albugineous tunic 660 digit 657, – blood supply 369 Acquired reflexes 63 – of penis 443, 446 – palmar – displacement 367, 715 Acrocoracohumeral ligament 750 – of testis 433 –– digital veins 665 – perigastric ring of arteries 370 Acromion 171, 173–174, 190 Albumen (egg white) 766 –– ligament of proximal interphalan- – projection onto lateral body wall 743 – fracture 727 Alpha cells 596 202 geal joint Absolute dullness 746 Acropodium 181, 244,259 Alular –– proper artery 727 Absorption of substances 67 ACTH (adrenocorticotropic – digit 748 –– proper artery of fourth digit Accessory hormone) 36, 588 – remiges 778, 778 660 657, – axillary lymph node 505, 507 Actin filaments 44 Alveolar –– proper nerve 727 – carpal bone 173, 182, 182–183,184, Adamantine 339 – border of mandible 90, 92 –– -

A Functional Perspective on the Evolution of the Cochlea
Downloaded from http://perspectivesinmedicine.cshlp.org/ on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press A Functional Perspective on the Evolution of the Cochlea Christine Köppl and Geoffrey A. Manley Cluster of Excellence “Hearing4all” and Research Centre Neurosensory Science, Department of Neuroscience, School of Medicine and Health Science, Carl von Ossietzky University Oldenburg, 26129 Oldenburg, Germany Correspondence: [email protected] This review summarizes paleontological data as well as studies on the morphology, function, and molecular evolution of the cochlea of living mammals (monotremes, marsupials, and placentals). The most parsimonious scenario is an early evolution of the characteristic organ of Corti, with inner and outer hair cells and nascent electromotility. Most remaining unique features, such as loss of the lagenar macula, coiling of the cochlea, and bony laminae supporting the basilar membrane, arose later, after the separation of the monotreme lineage, but before marsupial and placental mammals diverged. The question of when hearing sensitivity first extended into the ultrasonic range (defined here as >20 kHz) remains speculative, not least because of the late appearance of the definitive mammalian middle ear. The last significant change was optimizing the operating voltage range of prestin, and thus the efficiency of the outer hair cells’ amplifying action, in the placental lineage only. t is well known that the term cochlea derives structure of their cochleae can only be under- Ifrom the Greek word for snail. However, in the stood when the history of their lineages is taken auditory literature, its usage has long ceased to into account. Historical contingency has had an be strictly tied to a coiled shape and is often used enormous influence on these sensory systems to mean any auditory organ of a land vertebrate. -

The Ultrastructure of the Sensory Hairs And
THE ULTRASTRUCTURE OF THE SENSORY HAIRS AND ASSOCIATED ORGANELLES OF THE COCHLEAR INNER HAIR CELL, WITH REFERENCE TO DIRECTIONAL SENSITIVITY ARNDT J. DUVALL, 3RD, ~xKE FLOCK, and JAN WERSXLL Downloaded from http://rupress.org/jcb/article-pdf/29/3/497/1068042/497.pdf by guest on 29 September 2021 From the Depal~tment of Otolaryngology, Karolinska Sjukhuset and Gustav V Research Institute, Stockholm, Sweden. Dr. Duvall is a Visiting National Institutes of Health Fellow from the Uni- versity of Minnesota. Dr. Flock's present address is Bell Telephone Laboratories, Murray Hill, New Jersey ABSTRACT From the apical end of the inner hair cell of the organ of Corti in the guinea pig cochlea protrude four to five rows of stereocilia shaped in a pattern not unlike the wings of a bird. In the area devoid of cuticular substance facing toward the tunnel of Corti lies a consistently present centriole. The ultrastructure of this centriole is similar to that of the basal body of the kinocilium located in the periphery of the sensory hair bundles in the vestibular and lateral line organ sensory cells and to that of the centrioles of other cells. The physiological implications of the anatomical orientation of this centriole are discussed in terms of direc- tional sensitivity. INTRODUCTION The sensory hairs of the receptor cells of the sensory the recent electron microscope studies of Flock, epithelia of the inner ear and the lateral line organ Kimura, Lundquist, and Wers~ill (14) demon- constitute an important link in the transformation strated the lack of a kinocilium on the hair cells of mechanical stimuli into the electrophysiological of the cochlea, but the presence, in its place, of a responses of the sensory cells. -

A Scanning Electron Microscopic Study of the Sacculus and Lagena in the Ears of Fifteen Species of Teleost Fishes
A Scanning Electron Microscopic Study of the Sacculus and Lagena in the Ears of Fifteen Species of Teleost Fishes ARTHUR N. POPPER Department of Zoology and Laboratory of Sensory Sciences, Unioersity of Hawaii, Honolulu, Hawaii 96822 and Kresge Hearing Research Institute, University of Michigan Medical Center, Ann Arbor, Michigan 48109 ABSTRACT The ulstrastructure of the saccular and lagenar maculae were studied in 15 species of teleost fishes, using the scanning electron microscope. Particular attention was paid to hair cell orientation patterns, composition of the ciliary bundles on the hair cells, hair cell distributions, and supporting cell types. The hair cells on both otolithic organs are divided into several groups with all of the hair cells in each group oriented in the same direction. The posterior region of the saccular macula in all species had dorsally oriented hair cells on the dorsal half of the macula and ventrally oriented hair cells on the ventral half. The cells on the anterior end of the macula were oriented anteriorly and poste- riorly, with the posterior group, in most species, being on the dorsal half of the anterior region of the macula. There was considerable inter-specific variation upon this basic pattern. Inter-specific variation on the lagenar macula was considerably less than on the saccular macula. The basic pattern in all of the species includes one dorsal cell group and one ventral cell group. There are four more-or-less discrete ciliary bundles, each varying in the rela- tive size of the kinocilia and stereocilia. Intermediary forms were also observed, making it difficult to differentiate ciliary bundles in some instances. -
Lagenar Potentials of the Vocal Plainfin Midshipman Fish, Porichthys Notatus
Journal of Comparative Physiology A https://doi.org/10.1007/s00359-018-01314-0 ORIGINAL PAPER Lagenar potentials of the vocal plainfin midshipman fish, Porichthys notatus Brooke J. Vetter1 · Lane H. Seeley2 · Joseph A. Sisneros1,3,4 Received: 25 September 2018 / Revised: 19 December 2018 / Accepted: 26 December 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The plainfin midshipman fishPorichthys ( notatus) is a species of marine teleost that produces acoustic signals that are important for mediating social behavior. The auditory sensitivity of the saccule is well established in this species, but the sensitivity and function of the midshipman’s putative auditory lagena are unknown. Here, we characterize the auditory- evoked potentials from hair cells in the lagena of reproductive type I males to determine the frequency response and auditory sensitivity of the lagena to behaviorally relevant acoustic stimuli. Lagenar potentials were recorded from the caudal and medial region of the lagena, while acoustic stimuli were presented by an underwater speaker. Our results indicate that the midshipman lagena has a similar low-frequency sensitivity to that of the midshipman saccule based on sound pressure and acceleration (re: 1 µPa and 1 ms−2, respectively), but the thresholds of the lagena were higher across all frequencies tested. The relatively high auditory thresholds of the lagena may be important for encoding high levels of behaviorally relevant acoustic stimuli when close to a sound source. Keywords Auditory-evoked potentials · Lagena · Hair cell tuning · Hearing Introduction model organism for investigating the neural basis of acoustic communication, as this species provides a highly tractable Many teleost fishes have evolved adaptations for the pro- model for examining auditory-driven social behavior and duction and reception of social acoustic communication its underlying neurophysiology (Bass et al.