The Endocannabinoid System As a Target for Novel Anxiolytic Drugs
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Dissertation Entitled Uncovering Cannabinoid Signaling in C. Elegans
A Dissertation Entitled Uncovering Cannabinoid Signaling in C. elegans: A New Platform to Study the Effects of Medicinal Cannabis By Mitchell Duane Oakes Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology ________________________________________ Dr. Richard Komuniecki, Committee Chair _______________________________________ Dr. Bruce Bamber, Committee Member ________________________________________ Dr. Patricia Komuniecki, Committee Member ________________________________________ Dr. Robert Steven, Committee Member ________________________________________ Dr. Ajith Karunarathne, Committee Member ________________________________________ Dr. Jianyang Du, Committee Member ________________________________________ Dr. Amanda Bryant-Friedrich, Dean College of Graduate Studies The University of Toledo August 2018 Copyright 2018, Mitchell Duane Oakes This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Uncovering Cannabinoid Signaling in C. elegans: A New Platform to Study the Effects of Medical Cannabis By Mitchell Duane Oakes Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Doctor of Philosophy Degree in Biology The University of Toledo August 2018 Cannabis or marijuana, a popular recreational drug, alters sensory perception and exerts a range of medicinal benefits. The present study demonstrates that C. elegans exposed to -

The Endocannabinoid System: Critical for the Neurotrophic Action of Psychotropic Drugs
Biomedical Reviews 2010; 21: 31-46. © Bul garian Society for Cell Biology ISSN 1314-1929 THE ENDOCANNABINOID SYSTEM: CRITICAL FOR THE NEUROTROPHIC ACTION OF PSYCHOTROPIC DRUGS Parichehr Hassanzadeh Research Center for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran There is growing evidence that neurotrophins besides their well-established actions in regulating the survival, differentiation, and maintenance of the functions of specific populations of neurons, act as the potential mediators of antidepressant responses. Previous studies on the regulation of nerve growth factor (NGF) levels by psychotropic medications are limited in scope and the underlying mechanism(s) remain elusive. In this review, the latest findings on the effects of pharmacologically heterogeneous groups of psychotropic drugs on NGF contents in the brain regions involved in the modulation of emotions are summarized. Moreover, the therapeutic potentials of the endocannabinoid system which is linked to depression and/or antidepressant effects and appears to interact with neurotrophin signalling, are reviewed. New findings demonstrate that endocannabinoid system is involved in the mechanisms of action of certain psychotropic medications including neurokinin receptor antagonists and that these are mediated via the upregulation of brain regional levels of NGF. This provides a better understanding of the pathophysi- ological mechanisms underlying neuropsychiatric disorders, leading to novel drug designs. Biomed Rev 2010; 21: 31-46. Key words: endocannabinoids, NGF, psychotropics, brain INTRODUCTION glucocorticoid activity are involved in the pathophysiology Depression is a serious and widespread mental disorder with of depression (2-4). However, the monoamine-based anti- high relapse rate which is characterized by an array of dis- depressants do not fulfil the expectations in terms of onset turbances in emotional behavior, memory, neurovegetative of action, efficacy, and tolerability. -

Pharmacological Enhancement of Cannabinoid CB1 Receptor Activity Elicits an Antidepressant-Like Response in the Rat Forced Swim Test
European Neuropsychopharmacology 15 (2005) 593 – 599 www.elsevier.com/locate/euroneuro Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test Matthew N. Hill, Boris B. Gorzalka* Department of Psychology, University of British Columbia, Vancouver, 2136 West Mall, Canada V6T 1Z4 Received 15 October 2004; received in revised form 1 February 2005; accepted 22 March 2005 Abstract These experiments aimed to assess whether enhanced activity at the cannabinoid CB1 receptor elicits antidepressant-like effects. To examine this we administered 1 and 5 mg/kg doses of the endocannabinoid uptake inhibitor AM404; 5 and 25 Ag/kg doses of HU-210, a potent CB1 receptor agonist; 1, 2.5 and 5 mg/kg of oleamide, which elicits cannabinoidergic actions; 1 and 5 mg/kg doses of AM 251, a selective CB1 receptor antagonist, as well as 10 mg/kg desipramine (a positive antidepressant control) and measured the duration of immobility, during a 5-min test session of the rat Porsolt forced swim test. Results demonstrated that administration of desipramine reduced immobility duration by about 50% and that all of AM404, oleamide and HU-210 administration induced comparable decreases in immobility that were blocked by pretreatment with AM 251. Administration of the antagonist AM 251 alone had no effect on immobility at either dose. These data suggest that enhancement of CB1 receptor signaling results in antidepressant effects in the forced swim test similar to that seen following conventional antidepressant administration. D 2005 Elsevier B.V. and ECNP. All rights reserved. Keywords: Cannabinoid; Antidepressant; Forced swim test; Oleamide; Rat 1. -

Influence of the CB1 Cannabinoid Receptors on the Activity of the Monoaminergic System in the Behavioural Tests in Mice
Accepted Manuscript Title: Influence of the CB1 cannabinoid receptors on the activity of the monoaminergic system in the behavioural tests in mice Authors: Ewa Poleszak, Sylwia Wosko,´ Karolina Sławinska,´ Elzbieta˙ Wyska, Aleksandra Szopa, Urszula Doboszewska, Piotr Wlaz,´ Aleksandra Wlaz,´ Jarosław Dudka, Jarosław Szponar, Anna Serefko PII: S0361-9230(19)30298-9 DOI: https://doi.org/10.1016/j.brainresbull.2019.05.021 Reference: BRB 9698 To appear in: Brain Research Bulletin Received date: 16 April 2019 Revised date: 28 May 2019 Accepted date: 29 May 2019 Please cite this article as: Poleszak E, Wosko´ S, Sławinska´ K, Wyska E, Szopa A, Doboszewska U, WlazP,Wla´ z´ A, Dudka J, Szponar J, Serefko A, Influence of the CB1 cannabinoid receptors on the activity of the monoaminergic system in the behavioural tests in mice, Brain Research Bulletin (2019), https://doi.org/10.1016/j.brainresbull.2019.05.021 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Influence of the CB1 cannabinoid receptors on the activity of the monoaminergic system in the behavioural tests in mice Ewa Poleszak 1,*, Sylwia Wośko 1, Karolina Sławińska 1, Elżbieta Wyska -

The Endocannabinoid System
Neurobiology of Learning and Memory 112 (2014) 30–43 Contents lists available at ScienceDirect Neurobiology of Learning and Memory journal homepage: www.elsevier.com/locate/ynlme Review The endocannabinoid system: An emotional buffer in the modulation of memory function ⇑ Maria Morena a, Patrizia Campolongo a,b, a Department of Physiology and Pharmacology, Sapienza University of Rome, P.le A. Moro 5, 00185 Rome, Italy b Sapienza School of Advanced Studies, Sapienza University of Rome, P.le A. Moro 5, 00185 Rome, Italy article info abstract Article history: Extensive evidence indicates that endocannabinoids modulate cognitive processes in animal models and Received 10 July 2013 human subjects. However, the results of endocannabinoid system manipulations on cognition have been Revised 16 December 2013 contradictory. As for anxiety behavior, a duality has indeed emerged with regard to cannabinoid effects Accepted 20 December 2013 on memory for emotional experiences. Here we summarize findings describing cannabinoid effects on Available online 29 December 2013 memory acquisition, consolidation, retrieval and extinction. Additionally, we review findings showing how the endocannabinoid system modulates memory function differentially, depending on the level of Keywords: stress and arousal associated with the experimental context. Based on the evidence reviewed here, we Cannabinoids propose that the endocannabinoid system is an emotional buffer that moderates the effects of environ- Anandamide Memory modulation mental context and stress on cognitive processes. Emotional arousal Ó 2013 Elsevier Inc. All rights reserved. Stress 1. Introduction nabinoid effects on memory function, we propose hypotheses to explain the observed dual/opposing effects of cannabinoids on Emerging evidence indicates that cannabinoid drugs can induce emotional memory functions. -

The Role of Cannabinoidergic System in Prenatal Neurodevelopment Nima Naderi
Iranian Journal of Pharmaceutical Research (2013), 12 (2): 257-259 Copyright © 2013 by School of Pharmacy Shaheed Beheshti University of Medical Sciences and Health Services Editorial The Role of Cannabinoidergic System in Prenatal Neurodevelopment Nima Naderi The cannabinoidergic system acquired a reputation as the most abundant G-protein coupled receptor in the CNS that acts as retrograde modulator of neurotransmitter release. Recently, endocannabinoids (ECBs) have been highlighted as neurodevelopmental signaling cues that exert a regulatory role in brain development. The interruption in ECB system elements (including receptors and enzymes of synthesis and degradation) in the developing brain is in relation with postnatal CNS pathophysiology. The rapid rates of ECB synthesis/degradation reveal the existence of a dynamically regulated ECB tone during the active neurogenesis. The cannabinoid CB1 receptor is expressed from very early stages of embryonic development, even before the appearance of the neural tube and ectoderm development. CB1 is present in trophoblast stem cells and its deletion results in reduced cell proliferation and differentiation that is followed by aberrant formation of placenta and compromised embryo implantation. In addition to CB1 receptor expression, the other cannabinoid receptor, the CB2 receptor, is also present in the inner cell mass, and is involved in embryonic stem-derived hematopoietic cell proliferation and differentiation. In mammals, CB1 receptor expression is characterized by its abundant levels in white matter areas (where the axons of neural cells are present), with their levels progressively increasing from prenatal stages to adulthood in grey matter areas (where mostly occupied by neural cell bodies and dendrites) during neural development. The expression of CB1 receptor during the development occurs in active neurogenesis and axonal migration and prior to the synaptic maturation and neuronal activity. -

Effect of Interaction Between Acute Administration of Morphine
Iranian Journal of Basic Medical Sciences ijbms.mums.ac.ir Effect of interaction between acute administration of morphine and cannabinoid compounds on spontaneous excitatory and inhibitory postsynaptic currents of magnocellular neurons of supraoptic nucleus Mitra Yousefpour 1*, Nima Naderi 2, Fereshteh Motamedi 3, 4 1 Department of Physiology, Faculty of Medicine, Artesh University of Medical Science, Tehran, Iran 2 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran 3 Neuroscience Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran 4 Department of Physiology, Faculty of Medicine, Shahid Beheshti University of Medical Science, Tehran, Iran A R T I C L E I N F O A B S T R A C T Article type: Objective(s): Opioids and cannabinoids are two important compounds that have been shown to Original article influence the activity of magnocellular neurons (MCNs) of supraoptic nucleus (SON). The interaction between opioidergic and cannabinoidergic systems in various structures of the brain Article history: and spinal cord is now well established, but not in the MCNs of SON. Received: Apr 3, 2015 Materials and methods: In this study, whole cell patch clamp recording of neurons in rat brain slice Accepted: Apr 7, 2016 was used to investigate the effect of acute morphine and cannabinoid administration on spontaneous inhibitory and excitatory spostsynaptic currents (sIPSCs and sEPSCs) in MCNs. Keywords: Results: Bath application of morphine produced an increase in sEPSCs frequency and a decrease in Cannabinoid sIPSCs frequency. In contrast, bath application of URB597 (fatty acid amide hydrolase (FAAH) Morphine inhibitor) produced a decrease in sEPSCs frequency but an increase in sIPSCs frequency. -

Role of the Endocannabinoid System in Alcohol-Related Behaviors Basalingappa L
The Open Neuropsychopharmacology Journal, 2009, 2, 31-39 31 Open Access Role of the Endocannabinoid System in Alcohol-Related Behaviors Basalingappa L. Hungund*,1,2,3 and K. Vinod Yaragudri*,1,2,4 1New York State Psychiatric Institute, New York, USA 2Division of Analytical Psychopharmacology, Nathan Kline Institute for Psychiatric Research, Orangeburg, New York, USA 3Division of Analytical Psychopharmacology, Department of Psychiatry, College of Physicians & Surgeons, Columbia University, New York, USA 4Department of Child & Adolescent Psychiatry, New York University School of Medicine, New York, USA Abstract: Alcoholism is a psychiatric disorder characterized by impaired control over drinking, leading to tolerance, physical dependence, uncontrollable craving and relapse. The mechanism/s underlying this disorder is poorly understood at present. Ethanol (alcohol) effects are mediated through several signal transduction pathways involving many neurotransmitters and ion channels in various brain regions. There is a growing body of evidence now suggesting a critical role for the endocannabinoid (EC) system in alcohol-related behaviors. The EC system is comprised of endogenous cannabimimetic substances (endocannabinoids) and their receptors [cannabinoid (CB)] and the enzymes involved in the synthesis and degradation of the ECs. Recent studies have demonstrated that both the genetic and pharmacological manipulation of the EC system modulate the development of tolerance to and dependence on alcohol. The present article provides a review of the existing literature on the role of the EC system, and possible mechanisms and the therapeutic potential of the drugs targeted against this system in preventing alcohol addiction. Keywords: Alcoholism, anandamide, CB1 receptor, dopamine, nucleus accumbens. INTRODUCTION G-protein coupled receptors (GPCRs) have been cloned; the cannabinoid CB1 and CB2 [9]. -

Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects
International Journal of Molecular Sciences Article Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects 1, 2, 3 4 Qing-Rong Liu *, Ana Canseco-Alba y, Ying Liang , Hiroki Ishiguro and Emmanuel S. Onaivi 2,* 1 Laboratory of Clinical Investigation, National Institute on Aging, Baltimore, MD 21224, USA 2 Department of Biology, William Paterson University, Wayne, NJ 07470, USA; [email protected] 3 College of Food Science and Engineering, Central South University of Forestry and Technology, Changsha 410004, Hunan, China; [email protected] 4 Department of Neuropsychiatry, Graduate School of Medical Science, University of Yamanashi, Chuo, Yamanashi 409-3898, Japan; [email protected] * Correspondence: [email protected] (Q.-R.L.); [email protected] (E.S.O.) New address: Dirección de Investigación, Instituto Nacional de Neurología y Neurocirugía INNN, y Laboratorio de investigación en adicciones, La Fama, Tlalpan, Mexico City 14269, Mexico. Received: 1 November 2020; Accepted: 17 December 2020; Published: 21 December 2020 Abstract: There are two well-characterized cannabinoid receptors (CB1R and CB2R and other candidates): the central nervous system (CNS) enriched CB1R and peripheral tissue enriched CB2R with a wide dynamic range of expression levels in different cell types of human tissues. Hepatocytes and neurons express low baseline CB1R and CB2R, respectively, and their cell-type-specific functions are not well defined. Here we report inducible expression of CB1R in the liver by high-fat and high sugar diet and CB2R in cortical neurons by methamphetamine. While there is less controversy about hepatocyte CB1R, the presence of functional neuronal CB2R is still debated to date. -
![Upregulation of CB1 Receptors and Agonist-Stimulated [35S]](https://docslib.b-cdn.net/cover/6766/upregulation-of-cb1-receptors-and-agonist-stimulated-35s-5276766.webp)
Upregulation of CB1 Receptors and Agonist-Stimulated [35S]
Molecular Psychiatry (2004) 9, 184–190 &2004 Nature Publishing Group All rights reserved 1359-4184/04 $25.00 www.nature.com/mp ORIGINAL RESEARCH ARTICLE Upregulation of CB1 receptors and agonist-stimulated [35S]GTPcS binding in the prefrontal cortex of depressed suicide victims BL Hungund1,2,3,5, KY Vinod2,5, SA Kassir1, BS Basavarajappa1,2, R Yalamanchili2, TB Cooper1,2,3, JJ Mann1,3 and V Arango1,3,4 1New York State Psychiatric Institute, New York, NY, USA; 2Nathan Kline Institute for Psychiatric Research, Orangeburg, NY, USA; 3Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York, NY, USA; 4Department of Anatomy and Cell Biology, College of Physicians and Surgeons, Columbia University, New York, NY, USA Endogenous and exogenous cannabinoids (CBs) acting through the CB1 receptors have been implicated in the regulation of several behavioral and neuroendocrine functions. Modulation of endocannabinoidergic system by ethanol in mouse brain, and the association of suicide and mood disorders with alcoholism suggest possible involvement of the cannabinoidergic system in the pathophysiology of depression and suicide. Therefore, the present study was undertaken to examine the levels of CB1 receptors and mediated signaling in the dorsolateral prefrontal cortex (DLPFC) of subjects with major depression who had died by suicides 3 35 (depressed suicides, DS). [ H]CP-55,940 and CB1 receptor-stimulated [ S]GTPcS binding sites were analyzed in membranes obtained from DLPFC of DS (10) and matched normal controls (10). Upregulation (24%, Po0.0001) of CB1 receptor density (Bmax) was observed in DS (644.6748.8 fmol/mg protein) compared with matched controls (493.3752.7 fmol/mg protein). -
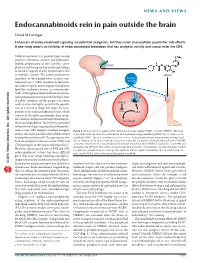
Endocannabinoids Rein in Pain Outside the Brain
NEWS AND VIEWS Endocannabinoids rein in pain outside the brain David M Lovinger Enhancers of endocannabinoid signaling are potential analgesics, but they cause unacceptable psychiatric side effects. A new study reports an inhibitor of endocannabinoid breakdown that has analgesic activity and cannot enter the CNS. Medical marijuana is a popular topic among scientists, clinicians, activists and politicians. Indeed, preparations of the Cannabis sativa BCRP plant are now being used in medicinal settings to stimulate appetite and for symptomatic relief in multiple sclerosis. The major psychoactive URB937 ingredient of these preparations activates can- Sensory neuron nabinoid type 1 (CB1) receptors in the brain, the same receptors that recognize endogenous lipid-like mediators known as endocannabi- noids. Although potential medicinal uses of can- nabis preparations have garnered the lion’s share CB1 of public attention, steady progress has been URB937 made in determining the potential therapeutic uses of a variety of drugs that target the com- ponents of the endocannabinoid system, which FAAH AEA consists of the endocannabinoids, their recep- tors and the enzymes involved in their biosyn- AA + EtH N thesis and degradation. Pain relief is a potential 2 indication for drugs targeting the endocannabi- noid system. CB1 receptors mediate analgesic Figure 1 Mechanism of analgesic action of the peripherally targeted FAAH inhibitor URB937. The drug actions and endocannabinoids modulate neural is excluded from the brain by a transporter with a pharmacology resembling BCRP, but is free to act on nociceptive mechanisms1,2. Receptors present in peripheral FAAH. The site of analgesic action is most likely peripheral pain-sensing nerve endings (red both the peripheral nervous system (PNS) and circle). -

NIH Public Access Author Manuscript Mini Rev Med Chem
NIH Public Access Author Manuscript Mini Rev Med Chem. Author manuscript; available in PMC 2007 September 11. NIH-PA Author Manuscript Published in final edited form as: Mini Rev Med Chem. 2007 August ; 7(8): 769–779. The endocannabinoid signaling system: a potential target for next-generation therapeutics for alcoholism Balapal S. Basavarajappa1,2,3,* 1 Division of Analytical Psychopharmacology, New York State Psychiatric Institute 2 Department of Psychiatry, College of Physicians & Surgeons, Columbia University, New York, NY 10032, USA 3 Nathan Kline Institute for Psychiatric Research, Orangeburg, Orangeburg, NY 10962, USA Abstract Research into the endocannabinoid signaling system has grown exponentially in recent years NIH-PA Author Manuscript following the discovery of cannabinoid receptors (CB) and their endogenous ligands, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Important advances have been made in our understanding of the endocannabinoid signaling system in various aspects of alcoholism, including alcohol-seeking behavior. Alcohol increases the synthesis or impairs the degradation of endocannabinoids, leading to a locally elevated endocannabinoid tone within the brain. Elevated endocannabinoid tone might be expected to result in compensatory down-regulation of CB1 receptors or dampened signal transduction. Following release, endocannabinoids diffuse back to the presynaptic neuron where they act as short-range modulators of synaptic activity by altering neurotransmitter release and synaptic plasticity. Mice treated with the CB1 receptor antagonist SR141716A (rimonabant) or homozygous for a deletion of the CB1 receptor gene exhibit reduced voluntary alcohol intake. CB1 knockout mice also show increased alcohol sensitivity, withdrawal, and reduced conditioned place preference. Conversely, activation of CB1 receptor promotes alcohol intake.