Ommastrephidae OMMASTREPHIDAE Arrow Squids, Flying Squids by M.C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

CEPHALOPODS 688 Cephalopods
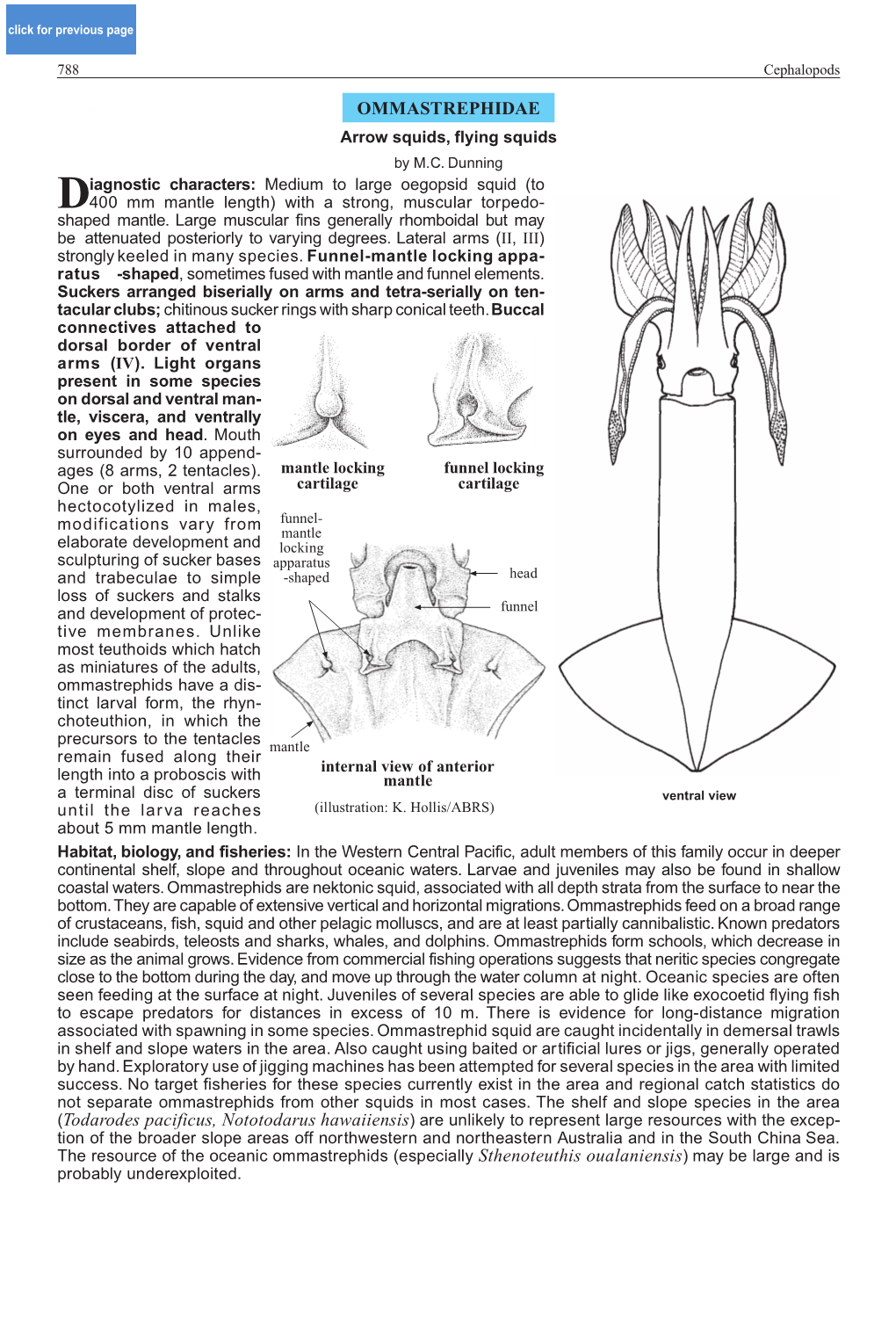
click for previous page CEPHALOPODS 688 Cephalopods Introduction and GeneralINTRODUCTION Remarks AND GENERAL REMARKS by M.C. Dunning, M.D. Norman, and A.L. Reid iving cephalopods include nautiluses, bobtail and bottle squids, pygmy cuttlefishes, cuttlefishes, Lsquids, and octopuses. While they may not be as diverse a group as other molluscs or as the bony fishes in terms of number of species (about 600 cephalopod species described worldwide), they are very abundant and some reach large sizes. Hence they are of considerable ecological and commercial fisheries importance globally and in the Western Central Pacific. Remarks on MajorREMARKS Groups of CommercialON MAJOR Importance GROUPS OF COMMERCIAL IMPORTANCE Nautiluses (Family Nautilidae) Nautiluses are the only living cephalopods with an external shell throughout their life cycle. This shell is divided into chambers by a large number of septae and provides buoyancy to the animal. The animal is housed in the newest chamber. A muscular hood on the dorsal side helps close the aperture when the animal is withdrawn into the shell. Nautiluses have primitive eyes filled with seawater and without lenses. They have arms that are whip-like tentacles arranged in a double crown surrounding the mouth. Although they have no suckers on these arms, mucus associated with them is adherent. Nautiluses are restricted to deeper continental shelf and slope waters of the Indo-West Pacific and are caught by artisanal fishers using baited traps set on the bottom. The flesh is used for food and the shell for the souvenir trade. Specimens are also caught for live export for use in home aquaria and for research purposes. -
Title NOTES on the OCCURRENCE and BIOLOGY of THE
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository NOTES ON THE OCCURRENCE AND BIOLOGY OF THE Title OCEANIC SQUID, THYSANOTEUTHIS RHOMBUS TROSCHEL, IN JAPAN Author(s) Nishimura, Saburo PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1966), 14(4): 327-349 Issue Date 1966-09-20 URL http://hdl.handle.net/2433/175443 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University NOTES ON THE OCCURRENCE AND BIOLOGY OF THE OCEANIC SQUID, THYSANOTEUTHIS 1 RHOMBUS TROSCHEL, IN JAPAN ) SABURO NISHIMURA Seto Marine Biological Laboratory, Sirahama With 6 Text-figures Though it is not so huge as Architeuthis or Moroteuthis nor so bizarre as Chiroteuthis or Opisthoteuthis, Thysanoteuthis rhombus TRoscHEL (Cephalopoda: Teuthoidea) is still one of the most remarkable members of the Japanese cephalopod fauna which com prises about one hundred and forty species. Its fully grown body will attain more than 80 em in mantle length or more than 19 kg in weight and its robust body with the enormously developed fins makes it quite distinct from all other teuthoidean cephalopods; these features seem to deserve well of its being called a noticeable creature in the ocean. This cephalopod is found rather frequently and in a moderate quantity in certain districts of Japan and well known to local fishermen by various Japanese names such as "taru-ika" (barrel squid), "hako-ika" (box squid), "sode-ika" (sleeved squid), "kasa ika" (umbrella squid), "aka-ika" (red squid), etc. However, it is apparently very scarce in other parts of the world, being recorded outside the Japanese waters so far only from the Mediterranean (TROSCHEL 1857; JATTA 1896; NAEF 1921-28; etc.), the waters around Madeira (REES & MAUL 1956) and the Cape of Good Hope (BARNARD 1934), and almost nothing is known of its life history including migration, behavior, life span, etc. -
<I>Sthenoteuthis Oualaniensis</I>
BULLETIN OF MARINE SCIENCE, 71(2): 1105–1108, 2002 THE AGE AND GROWTH OF STHENOTEUTHIS OUALANIENSIS (CEPHALOPODA: OMMASTREPHIDAE) IN THE PACIFIC OCEAN Kaori Takagi, Takeru Kitahara, Naoki Suzuki, Junta Mori and Akihiko Yatsu Sthenoteuthis oualaniensis is distributed in the tropical and subtropical areas of the Pacific and the Indian Oceans. According to Nesis (1993), there is a complex population structure in S. oualaniensis, as is the case in many other ommastrephids and some loliginids. In the Pacific Ocean, there is the middle-sized squid which is a widespread and typical one (Nesis, 1993). Arkhipkin and Bizikov (1991) examined the statoliths of middle-sized female in the Indian Ocean and determined its growth. S. oualaniensis is, though, one of the most difficult species in the Ommastrephidae for the observation of statolith incre- ments due to the numerous occulting crystals and weak contrast in the increments (Uozumi, 1993). Using a newly developed heating technique in processing statoliths, we estimated the age and growth of S. oualaniensis, assuming the daily deposition of increments. MATERIALS AND METHODS Samples of S. oualaniensis were collected between September and December 1993 in the Pa- cific Ocean around the Hawaii and the Ogasawara (Bonin) Islands. We used the statoliths of 53 adults (112–284 mm in mantle length (ML), 21 males and 32 females) and 112 paralarvae (0.7– 13.5 mm in ML). The adults were captured by drift nets and jigs. The paralarvae were captured by bongo nets and a larval net. To examine the relationship between ML and age, we also used 6 other juveniles (39–50 mm in ML) captured using a dip net. -

Redalyc.Subcutaneous Photophores in the Jumbo Squid Dosidicus Gigas
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Lohrmann, Karin B. Subcutaneous photophores in the jumbo squid Dosidicus gigas (d'Orbigny, 1835) (Cephalopoda: Ommastrephidae) Revista de Biología Marina y Oceanografía, vol. 43, núm. 2, agosto, 2008, pp. 275-284 Universidad de Valparaíso Viña del Mar, Chile Disponible en: http://www.redalyc.org/articulo.oa?id=47943205 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Revista de Biología Marina y Oceanografía 43(2): 275-284, agosto de 2008 Subcutaneous photophores in the jumbo squid Dosidicus gigas (d’Orbigny, 1835) (Cephalopoda: Ommastrephidae) Fotóforos subcutáneos en el calamar gigante Dosidicus gigas (d’Orbigny, 1835) (Cephalopoda: Ommastrephidae) Karin B. Lohrmann1 1Facultad de Ciencias del Mar, Universidad Católica del Norte, Coquimbo, Chile. Larrondo 1281, Coquimbo, Chile [email protected] Resumen.- En Dosidicus gigas se observaron pequeñas Abstract.- In Dosidicus gigas small pale yellow ovoid inclusiones de color amarillo pálido embebidas a distintas inclusion bodies corresponded to subcutaneous photophores, profundidades en el músculo del manto, las que corresponden which were embedded in the mantle muscle, at differing depths. a fotóforos. A nivel histológico los fotóforos están formados At the histological level the photophores were composed of a por un tejido fotogenerador, que se tiñe de color naranja intenso photogenic tissue, which stained bright orange with Mallory con tinción tricrómica de Mallory y un tejido vacuolar, que lo triple stain. -

Length-Weight Relationship of Neon Flying Squid Ommastrephes Bartramii (Cephalopoda: Ommastrephidae) Caught from Indian Sector of Southern Ocean
Indian Journal of Geo-Marine Science Vol. 43(8), August 2014, pp 1581-1584 Length-weight relationship of neon flying squid Ommastrephes bartramii (Cephalopoda: Ommastrephidae) caught from Indian sector of Southern Ocean. *Aneesh Kumar K. V1#., Pravin P1., Ragesh N2 & Meenakumari B3. 1Central Institute of Fisheries Technology, Matsyapuri, Willingdon Island. Cochin-682029, India, 2Central Marine Fisheries Research Institute, Cochin- 682018, India, 3Indian Council of Agricultural Research, Krishi Anusandhan Bhavan 2, New Delhi-110012, India #Present address: Centre for Marine Living Resources and Ecology Kendriya Bhavan, CSEZ P.O., Cochin-682037, India *[E. Mail: [email protected]] Received 1 July 2013; revised 7 August 2013 Length-weight relationship of the Neon flying squid Ommastrephes bartramii, caught from the Indian Sector of Southern Ocean was estimated as male W= 0.0235 L 3.05 (R2 = 0.990719) and females W= 0.0283 L 2.99 (R2 = 0.919944). The species follows an isometric growth pattern and no significant difference was observed between both sexes. [Key words: Length- Weight Relation, Squid, Ommastrephes bartramii, Southern Ocean] Introduction Ommastrephes bartramii (Lesueur, 1821) is a morphometric characters gives a better idea for widely distributed oceanic ommastrephid species understanding the relationship between the species throughout the subtropical and temperate waters of and to compare same species in different both northern and southern hemisphere and geographical areas8. The study of the individual excluded from the equatorial waters of all three growth pattern gives an insight about the population oceans1 and forms a major fishery in the Japanese dynamics of the species such as growth and squid fisheries in the Pacific Ocean2. -

Forage Fish Management Plan
Oregon Forage Fish Management Plan November 19, 2016 Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship to Other State Policies ......................................................................................................... 7 Public Process Developing this Plan .......................................................................................................... 8 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis .................................................................................................................................... -

Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1
Pacific Science (1995), vol. 49, no. 2: 143-155 © 1995 by University of Hawai'i Press. All rights reserved Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1 RICHARD EDWARD YOUNG 2 ABSTRACT: Pelagic cephalopods of the mesopelagic-boundary region in Hawai'i have proven difficult to sample but seem to occupy a variety ofhabitats within this zone. Abralia trigonura Berry inhabits the zone only as adults; A. astrosticta Berry may inhabit the inner boundary zone, and Pterygioteuthis giardi Fischer appears to be a facultative inhabitant. Three other mesopelagic species, Liocranchia reinhardti (Steenstrup), Chiroteuthis imperator Chun, and Iridoteuthis iris (Berry), are probable inhabitants; the latter two are suspected to be nonvertical migrants. The mesopelagic-boundary region also contains a variety of other pelagic cephalopods. Some are transients, common species of the mesopelagic zone in offshore waters such as Abraliopsis spp., neritic species such as Euprymna scolopes Berry, and oceanic epipelagic species such as Tremoctopus violaceus Chiaie and Argonauta argo Linnaeus. Others are appar ently permanent but either epipelagic (Onychoteuthis sp. C) or demersal (No totodarus hawaiiensis [Berry] and Haliphron atlanticus Steenstrup). Submersible observations show that Nototodarus hawaiiensis commonly "sits" on the bot tom and Haliphron atlanticus broods its young in the manner of some pelagic octopods. THE CONCEPT OF the mesopelagic-boundary over bottom depths of the same range. The region (m-b region) was first introduced by designation of an inner zone is based on Reid et al. (1991), although a general asso Reid'sfinding mesopelagic fishes resident there ciation of various mesopelagic animals with during both the day and night; mesopelagic land masses has been known for some time. -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

Squid and Octopus
Sector Improvement Profile: Squid and Octopus More than half (52%) of global production is yellow- or red-rated, indicating that improvements are needed, and 45% is status unknown. Our improvement efforts prioritize: • The 27% of global squid and octopus production that is red-rated; and • The 39% within the scope of Sustainable Fisheries Partnership’s Target 75 Initiative. Red-rated Species Source country Argentine shortfin squid Argentina Common octopus Mexico, Portugal, Spain Common squids nei Indonesia, Thailand Japanese flying squid Japan Mexican four-eyed octopus Mexico Octopuses nei Indonesia, Mauritania, Morocco, Philippines Various squids nei China, India The Monterey Bay Aquarium’s Seafood Watch Program ratings are based on specific location and production method information and make exceptions for specific brands. For detailed information, visit the Seafood Watch recommendations on squid and octopus. FIP or AIP Where available, the links below lead to detailed information about FIPs listed on FisheryProgress.org. Species Source country Day Octopus Madagascar California Two-Spot Octopus Mexico Giant Pacific Octopus Japan Japanese Flying Squid China Jumbo Squid Mexico, Peru Mitre Squid China Red octopus Chile, Mexico Octopus China The Seafood Certification & Ratings Collaboration unites five global seafood certification and ratings programs working together to coordinate our tools and increase our impact so that more seafood producers move along a clear path toward environmental sustainability and social responsibility. Learn -

Learning About Our Favourite Squid Species
Cephalopod Science Investigations LEARNING ABOUT OUR FAVOURITE SQUID SPECIES By Cushla Dromgool-Regan Eimear Manning & Anna Quinn www.EXPLORERS.ie The Explorers Education Programme is funded by the Marine Institute Explorers Education Programme engage with primary schools, teachers and children, creating marine leaders and ocean champions. The Explorers Education Programme team provides engaging activities, resources and support for teachers, children and the education network, delivering ocean literacy to primary schools. We aim to inspire children and educators to learn about our marine and maritime identity and heritage, as well as making informed and responsible decisions regarding the ocean and its resources. We communicate about the ocean in a meaningful way, increasing the awareness and understanding of our marine biodiversity, the environment, as well as the opportunities and social benefits of our ocean wealth. To help inspire children learning about the ocean, we have developed a series of teaching materials and resources about Squid! Check out our Explorers books: Cephalopod Science Investigations – Learning about Squid 101; My CSI Squid Workbook. Also, see our interactive film: Cephalopod Science Investigations – Learning about Squid 101 and Dissection. For more information about our Squid series see www.explorers.ie CEPHALOPOD SCIENCE INVESTIGATIONS LEARNING ABOUT OUR FAVOURITE SQUID SPECIES AUTHORS Cushla Dromgool-Regan Eimear Manning Anna Quinn PUBLISHED BY Marine Institute First published in 2021 Marine Institute, Rinville, Oranmore, Galway All or parts of the content of this publication may be reproduced without further permission for education purposes, provided the author and publisher are acknowledged. Authors: Cushla Dromgool-Regan, The Camden Education Trust; Eimear Manning, The Camden Education Trust; & Anna Quinn, Galway Atlantaquaria. -

Influence of Environmental Factors on Population Structure of Arrow Squid Nototodarus Gouldi: Implications for Stock Assessment
INFLUENCE OF ENVIRONMENTAL FACTORS ON POPULATION STRUCTURE OF ARROW SQUID NOTOTODARUS GOULDI: IMPLICATIONS FOR STOCK ASSESSMENT COREY PAUL GREEN, BAPPSC (FISHERIES) SUBMITTED IN FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF TASMANIA OCTOBER 2011 Arrow squid Nototodarus gouldi (McCoy, 1888) (Courtesy of Robert Ingpen, 1974) FRONTISPIECE DECLARATION STATEMENT OF ORIGINALITY This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of the my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. ………………………………………….…. 28th October 2011 Corey Paul Green Date AUTHORITY OF ACCESS This thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968. ………………………………………….…. 28th October 2011 Corey Paul Green Date I ACKNOWLEDGEMENTS This thesis assisted in fulfilling the objectives of the Fisheries Research and Development Corporation Project No. 2006/012 ―Arrow squid — stock variability, fishing techniques, trophic linkages — facing the challenges‖. Without such assistance this thesis would not have come to fruition. Research on statolith element composition was kindly funded by the Holsworth Wildlife Research Endowment (HWRE), and provided much information on arrow squid lifecycles. The University of Tasmania (UTAS), the Victorian Marine Science Consortium (VMSC) and the Department of Primary Industries — Fisheries Victoria, assisted in providing laboratories, desks and utilities, as well as offering a wonderful and inviting working environment.