Effect of Appendicularians and Copepods on Bacterioplankton Composition and Growth in the English Channel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
UNITED NATIONS ENVIRONMENT PROGRAM MEDITERRANEAN ACTION PLAN REGIONAL ACTIVITY CENTRE FOR SPECIALLY PROTECTED AREAS National monitoring program for biodiversity and non-indigenous species in Egypt PROF. MOUSTAFA M. FOUDA April 2017 1 Study required and financed by: Regional Activity Centre for Specially Protected Areas Boulevard du Leader Yasser Arafat BP 337 1080 Tunis Cedex – Tunisie Responsible of the study: Mehdi Aissi, EcApMEDII Programme officer In charge of the study: Prof. Moustafa M. Fouda Mr. Mohamed Said Abdelwarith Mr. Mahmoud Fawzy Kamel Ministry of Environment, Egyptian Environmental Affairs Agency (EEAA) With the participation of: Name, qualification and original institution of all the participants in the study (field mission or participation of national institutions) 2 TABLE OF CONTENTS page Acknowledgements 4 Preamble 5 Chapter 1: Introduction 9 Chapter 2: Institutional and regulatory aspects 40 Chapter 3: Scientific Aspects 49 Chapter 4: Development of monitoring program 59 Chapter 5: Existing Monitoring Program in Egypt 91 1. Monitoring program for habitat mapping 103 2. Marine MAMMALS monitoring program 109 3. Marine Turtles Monitoring Program 115 4. Monitoring Program for Seabirds 118 5. Non-Indigenous Species Monitoring Program 123 Chapter 6: Implementation / Operational Plan 131 Selected References 133 Annexes 143 3 AKNOWLEGEMENTS We would like to thank RAC/ SPA and EU for providing financial and technical assistances to prepare this monitoring programme. The preparation of this programme was the result of several contacts and interviews with many stakeholders from Government, research institutions, NGOs and fishermen. The author would like to express thanks to all for their support. In addition; we would like to acknowledge all participants who attended the workshop and represented the following institutions: 1. -
Appendicularia of CTAW
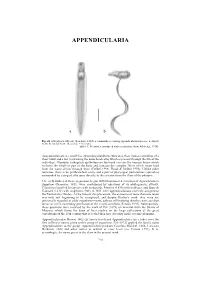
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Canestro 07Devbio Oikopleura Retinoic Acid Chordate.Pdf
Developmental Biology 305 (2007) 522–538 www.elsevier.com/locate/ydbio Development of a chordate anterior–posterior axis without classical retinoic acid signaling ⁎ Cristian Cañestro, John H. Postlethwait Institute of Neuroscience, University of Oregon, Eugene, OR 97403, USA Received for publication 28 November 2006; revised 18 February 2007; accepted 26 February 2007 Available online 2 March 2007 Abstract Developmental signaling by retinoic acid (RA) is thought to be an innovation essential for the origin of the chordate body plan. The larvacean urochordate Oikopleura dioica maintains a chordate body plan throughout life, and yet its genome appears to lack genes for RA synthesis, degradation, and reception. This suggests the hypothesis that the RA-machinery was lost during larvacean evolution, and predicts that Oikopleura development has become independent of RA-signaling. This prediction raises the problem that the anterior–posterior organization of a chordate body plan can be developed without the classical morphogenetic role of RA. To address this problem, we performed pharmacological treatments and analyses of developmental molecular markers to investigate whether RA acts in anterior–posterior axial patterning in Oikopleura embryos. Results revealed that RA does not cause homeotic posteriorization in Oikopleura as it does in vertebrates and cephalochordates, and showed that a chordate can develop the phylotypic body plan in the absence of the classical morphogenetic role of RA. A comparison of Oikopleura and ascidian evidence suggests that the lack of RA-induced homeotic posteriorization is a shared derived feature of urochordates. We discuss possible relationships of altered roles of RA in urochordate development to genomic events, such as rupture of the Hox-cluster, in the context of a new understanding of chordate phylogeny. -

Study of Dental Fluorosis in Subjects Related to a Phosphatic Fertilizer
Indian Journal of Geo Marine Sciences Vol. 46 (07), July 2017, pp. 1371-1380 Diversity and abundance of epipelagic larvaceans and calanoid copepods in the eastern equatorial Indian Ocean during the spring inter-monsoon Kaizhi Li, Jianqiang Yin*, Yehui Tan, Liangmin Huang & Gang Li Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China *[E-mail: [email protected]] Received 20 August 2015; revised 29 September 2015 This study investigated the species composition, distribution and abundance of larvaceans and calanoid copepods in the eastern equatorial Indian Ocean. In total, 25 species of larvaceans and 69 species of calanoid copepods were identified in the study area. Although the average diversity and evenness indexes of larvaceans were lower than those of calanoid copepods, the abundance of larvaceans was higher than that of calanoids with means of 40.1±14.9 ind m-3 and 28.4±9.1 ind m-3, respectively. Larvacean community was numerically dominated by Oikopleura fusiformis, Oikopleura longicauda, Oikopleura cophocerca, Fritillaria formica and Fritillaria pellucida, accounting for 83% of total larvacean abundance. The calanoid community was dominated by the following five species, represented 61% of calanoid copepods: Clausocalanus furcatus, Clausocalanus farrani, Acartia negligens, Acrocalanus longicornis as well as the copepodite stage of Euchaeta spp. This study highlights that the importance of larvaceans in the eastern equatorial Indian Ocean. [Keywords: Appendicularians, Calanoids, Water mass, Monsoon, Indian Ocean] Introduction It has been clear that small organisms of marine Clausocalanus) and the cyclopoid genera (such as zooplankton have historically been under-sampled by Oithona, Oncaea and Corycaeus1). -

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
National monitoring program for biodiversity and non-indigenous species in Egypt January 2016 1 TABLE OF CONTENTS page Acknowledgements 3 Preamble 4 Chapter 1: Introduction 8 Overview of Egypt Biodiversity 37 Chapter 2: Institutional and regulatory aspects 39 National Legislations 39 Regional and International conventions and agreements 46 Chapter 3: Scientific Aspects 48 Summary of Egyptian Marine Biodiversity Knowledge 48 The Current Situation in Egypt 56 Present state of Biodiversity knowledge 57 Chapter 4: Development of monitoring program 58 Introduction 58 Conclusions 103 Suggested Monitoring Program Suggested monitoring program for habitat mapping 104 Suggested marine MAMMALS monitoring program 109 Suggested Marine Turtles Monitoring Program 115 Suggested Monitoring Program for Seabirds 117 Suggested Non-Indigenous Species Monitoring Program 121 Chapter 5: Implementation / Operational Plan 128 Selected References 130 Annexes 141 2 AKNOWLEGEMENTS 3 Preamble The Ecosystem Approach (EcAp) is a strategy for the integrated management of land, water and living resources that promotes conservation and sustainable use in an equitable way, as stated by the Convention of Biological Diversity. This process aims to achieve the Good Environmental Status (GES) through the elaborated 11 Ecological Objectives and their respective common indicators. Since 2008, Contracting Parties to the Barcelona Convention have adopted the EcAp and agreed on a roadmap for its implementation. First phases of the EcAp process led to the accomplishment of 5 steps of the scheduled 7-steps process such as: 1) Definition of an Ecological Vision for the Mediterranean; 2) Setting common Mediterranean strategic goals; 3) Identification of an important ecosystem properties and assessment of ecological status and pressures; 4) Development of a set of ecological objectives corresponding to the Vision and strategic goals; and 5) Derivation of operational objectives with indicators and target levels. -

Species Composition and Horizontal Distribution of the Appendicularian Community in Waters Adjacent to the Kuroshio in Winter–Early Spring
Plankton Benthos Res 3(3): 152–164, 2008 Plankton & Benthos Research © The Plankton Society of Japan Species composition and horizontal distribution of the appendicularian community in waters adjacent to the Kuroshio in winter–early spring KIYOTAKA HIDAKA* National Research Institute of Fisheries Science, Fisheries Research Agency, 2–12–4 Fukuura, Kanazawa, Yokohama, Kanagawa, 236–8648 Japan Received 10 December 2007; Accepted 10 April 2008 Abstract: Species composition and horizontal distribution of appendicularians were investigated in waters adjacent to the Kuroshio, south of Honshu in winter–early spring. Twenty-one species belonging to 5 genera were found and the species composition was characterized by a strong dominance of Oikopleura longicauda and occasional dense occur- rence of Fritillaria pellucida. Oikopleura longicauda was distributed rather uniformly throughout the study area while F. pellucida was usually only sparsely distributed, although it was sometimes found in dense concentrations at the sta- tions close to the Kuroshio axis. Occurrence of O. dioica, a well-studied neritic species, was limited to the stations with a relatively shallow bottom and its relative importance in terms of abundance was small. Generally, the appendicularian biomass was less than one tenth of the copepod biomass in carbon. Nevertheless, the role of appendicularians in terms of secondary production was estimated to be smaller but comparable in magnitude to that of copepods, 1.7–202.3 mgC mϪ2 dϪ1 in the former and 30.0–185.2 mgC mϪ2 dϪ1 in the latter, because of their higher somatic growth rates and house production. Clearance rate of appendicularians was also estimated to be comparable to copepods, 3.1–591.3 L mϪ2 hϪ1 in the former and 49.0–462.1 L mϪ2 hϪ1 in the latter, owing to their much higher weight specific clearance rates. -

Spatial Distribution Patterns of Appendicularians in the Drake Passage: Potential Indicators of Water Masses?
diversity Article Spatial Distribution Patterns of Appendicularians in the Drake Passage: Potential Indicators of Water Masses? Marcin Kalarus 1 and Anna Panasiuk 2,* 1 Department of Aquatic Ecology, Maritime Institute, Gdynia Maritime University, Smoluchowskiego 1/3, 80-214 Gda´nsk,Poland; [email protected] 2 Department of Marine Plankton Research, Faculty of Oceanography and Geography, Institute of Oceanography, University of Gda´nsk,Av. Marszałka J. Piłsudskiego 46, 81-378 Gdynia, Poland * Correspondence: [email protected]; Tel.: +485-8523-6844 Abstract: Appendicularians are one of the most common animals found within zooplankton as- semblages. They play a very important role as filter feeders but are, unfortunately, inconsistently reported in the Antarctic literature. The present paper attempts to describe the zonal diversity of appendicularians and the main environmental factors influencing their communities in the Drake Passage. Samples were collected during Antarctic summer in 2009–2010. A total of eight species of larvaceans were identified. Fritillaria borealis was the species found in the highest numbers in almost the entire studied area, and was observed at all sampling stations. The distributions of other taxa were limited to specific hydrological zones and hydrological conditions. F. fraudax and Oikopleura gaussica were typical of the areas between the Polar Front and the Subantarctic Front zones, and their distributions were significantly correlated with temperature and salinity, likely making them good indicator species. The F. fusiformis distribution was strictly related to South American waters. In summary, temperature was the strongest environmental factor influencing the larvacean community structure in the Drake Passage, and we also found that testing environmental factors on larvaceans Citation: Kalarus, M.; Panasiuk, A. -

Redalyc.Appendicularian Distribution and Diversity in the Southern Gulf of Mexico
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Flores-Coto, César; Sanvicente-Añorve, Laura; Sánchez-Ramírez, Marina Appendicularian distribution and diversity in the southern Gulf of Mexico Revista Mexicana de Biodiversidad, vol. 81, núm. 1, abril, 2010, pp. 123-131 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42515998017 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 81: 123- 131, 2010 Appendicularian distribution and diversity in the southern Gulf of Mexico Distribución y diversidad de apendicularias en el sur del golfo de México César Flores-Coto1*, Laura Sanvicente-Añorve1 and Marina Sánchez-Ramírez2 1Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Circuito Exterior, Cd. Universitaria, 04510, México, D.F., México. 2Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional. Prolongación de Carpio S/N, 11340 México, D.F., México. *Correspondent author: [email protected] Abstract. The diversity and distribution of appendicularians on the continental shelf and upper part of the oceanic sea in the southern Gulf of Mexico is analyzed here for the fi rst time. Samples were collected in September 2003 using a fi ne mesh net. Twenty species were identifi ed, of which Fritillaria venusta and Pelagopleura oppressa are fi rst records for the Gulf of Mexico. Oikopleura species occur throughout the area, with greatest abundances in the upwelling waters of the inner shelf off Yucatán and Campeche. -

Oikopleura Dioica
To: [email protected] o ABSTRACT We propose that a BAC library should be constructed for the larvacean urochordate Oikopleura dioica. This animal may be the most similar among extant animals to the last common ancestor of all chordates, and thus, its investigation can illuminate the mechanisms causing the vertebrate genome expansion, the principles that guide the evolution of functions of duplicate genes in development, and the origin of vertebrate developmental innovations. Oikopleura dioica has a genome of about 70 Mb, the smallest of any chordate. o IMPORTANCE OF THE ORGANISM TO BIOMEDICAL OR BIOLOGICAL RESEARCH. The Animal. Oikopleura dioica is a 3 mm long urochordate, an abundant filter-feeder in marine plankton. Oikopleura and other members of its phylogenetic class, the larvaceans (or appendicularians) remain free swimming and tadpole-like into sexual maturity. Because larvaceans are basally diverging among the urochordates, this free-swimming life style may have been ancestral (Wada 1998). Larvaceans have more functional organ systems than do ascidian larvae; their embryos, tadpole larvae, and adults are transparent, which facilitates observations on all aspects of development (Fig. 1); embryonic development takes only 14 hours; and the generation time is less than two weeks. Furthermore, Oikopleura dioica is readily available from plankton tows for much of the year off Northern hemisphere coasts. Oikopleura dioica can be among the most abundant animals in offshore waters, even more than copepods, amounting to several animals per liter (Dagg & Ortner 1994; Capitano & Esnal 1998). With Oikopleura, one can perform single-pair matings, and culture the offspring in the lab continuously in the presence of fresh sea water (Fenaux & Gorsky, 1983; Bassham, unpubl). -

Integrating Developmental Clocking and Growth in a Life-History Model for the Planktonic Chordate Oikopleura Dioica
MARINE ECOLOGY PROGRESS SERIES Vol. 318: 81–88, 2006 Published August 3 Mar Ecol Prog Ser OPENPEN ACCESSCCESS Integrating developmental clocking and growth in a life-history model for the planktonic chordate Oikopleura dioica Dag L. Aksnes1,*, Christofer Troedsson2, Eric M. Thompson2 1Department of Biology, University of Bergen, PO Box 7800, 5020 Bergen, Norway 2Sars International Centre for Marine Molecular Biology, Thormøhlensgaten 55, 5008 Bergen, Norway ABSTRACT: We present a novel modelling approach applied to the appendicularian Oikopleura dioica. Growth and development are represented as separate processes, and a developmental clock is assumed to regulate development. Temperature influences the organism globally through aging and not separately through individual physiological processes as commonly applied in ecological models, and physiological rates are outputs rather than model inputs. The model accounts for devel- opment, growth, metabolic rates and life-history characteristics, with relatively few equations and parameters. Parameter values needed for simulations were derived from published data on the devel- opment of Caenorhabditis elegans and O. dioica. Comparisons of simulated generation times, respi- ration rates, assimilation rates and growth rates with independent data demonstrated predictive capability, but also raised inconsistencies that deserve future experimentation. The present model frames several testable predictions concerning development, growth and metabolic rates in multi- cellular organisms. Specifically, it suggests that the allometric scaling of metabolism to temperature and body mass should change when food limitation slows growth but not development. KEY WORDS: Oikopleura · Life history · Growth · Development · Aging · Allometry Resale or republication not permitted without written consent of the publisher INTRODUCTION ca (Ganot & Thompson 2002, Troedsson et al. -

DEIDRE MICHELLE GIBSON Feeding Growth and Reproduction Rates Of
DEIDRE MICHELLE GIBSON Feeding Growth and Reproduction Rates of the Doliolid Dolioletta gegenbauri Uljanin (Tunicata, Thaliacea) (Under the Direction of GUSTAV ADOLF PAFFENHÖFER) The objectives were to quantify feeding and growth of Dolioletta gegenbauri gonozooids and reproduction rates of Dolioletta gegenbauri phorozooids at 4 food concentrations, 3 food sizes, and 4 temperatures in order to determine their impact as grazers throughout the water column. Laboratory experiments were conducted at 16.5, 20, 23.5, and 26.5ºC quantifying removal of a 50:50 volumetric concentration of Thalassiosira weissflogii and Rhodomonas sp. at four different food concentrations of 7 20, 60, 160, and 390 µg C · l -1. Gonozooid clearance rates are similar at concentrations from 20 to 60 µg C · l -1, and decrease as the food concentrations increase to 160, and 390 µg C · l –1. The ingestion rates increase over a range of phytoplankton concentrations from 20 to 160 µg C · l -1, then decrease when abnormally high concentrations of 390 µg C · l -1 are offered. Clearance and ingestion rates increase as temperature increases from 16.5 to 26.5º C. The exponential growth rates range from k = 0.2 - 0.7 with the lowest rates occurring at the highest food concentration. Growth rates increase with increasing temperature from K = 0.1-0.3/day at 16.5º C to 0.45-0.7 at 26.5º C. Phorozooid release rates increased at 20 oC as concentration increases from 7 to 160 µg C/l. Release rates remained similar as the temperature increased from 16.5 to 26.5 oC at a phytoplankton concentration of 60 µg C/l. -

PDF Linkchapter
INDEX* With the exception of Allee el al. (1949) and Sverdrup et at. (1942 or 1946), indexed as such, junior authors are indexed to the page on which the senior author is cited although their names may appear only in the list of references to the chapter concerned; all authors in the annotated bibliographies are indexed directly. Certain variants and equivalents in specific and generic names are indicated without reference to their standing in nomenclature. Ship and expedition names are in small capitals. Attention is called to these subindexes: Intertidal ecology, p. 540; geographical summary of bottom communities, pp. 520-521; marine borers (systematic groups and substances attacked), pp. 1033-1034. Inasmuch as final assembly and collation of the index was done without assistance, errors of omis- sion and commission are those of the editor, for which he prays forgiveness. Abbott, D. P., 1197 Acipenser, 421 Abbs, Cooper, 988 gUldenstUdti, 905 Abe, N.r 1016, 1089, 1120, 1149 ruthenus, 394, 904 Abel, O., 10, 281, 942, 946, 960, 967, 980, 1016 stellatus, 905 Aberystwyth, algae, 1043 Acmaea, 1150 Abestopluma pennatula, 654 limatola, 551, 700, 1148 Abra (= Syndosmya) mitra, 551 alba community, 789 persona, 419 ovata, 846 scabra, 700 Abramis, 867, 868 Acnidosporidia, 418 brama, 795, 904, 905 Acoela, 420 Abundance (Abundanz), 474 Acrhella horrescens, 1096 of vertebrate remains, 968 Acrockordus granulatus, 1215 Abyssal (defined), 21 javanicus, 1215 animals (fig.), 662 Acropora, 437, 615, 618, 622, 627, 1096 clay, 645 acuminata, 619, 622; facing