A Proposition 65 Risk Evaluation of Lead in @@ Guitar Strings
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Safety Data Sheet
Safety Data Sheet TIN COATED PHOSPHOR BRONZE (Olin Brass SDS No: 01334.0001) EMERGENCY PHONE: 1-618-258-5167 This product consists of a base metal alloy coated with another metal. Attached are Safety Data Sheets (SDS) for the following metal products: Base Metal – >99% - Phosphor Bronze Coating – <1% - Tin Alloy THIS SAFETY DATA SHEET (SDS) KIT HAS BEEN PREPARED IN COMPLIANCE WITH THE FEDERAL OSHA HAZARD COMMUNICATION STANDARD, 29 CFR 1910.1200. THE INFORMATION IN THE ENCLOSED SDSs SHOULD BE PROVIDED TO ALL WHO WILL USE, HANDLE, STORE, TRANSPORT, OR OTHERWISE BE EXPOSED TO THIS PRODUCT. THIS INFORMATION HAS BEEN PREPARED FOR THE GUIDANCE OF PLANT ENGINEERING, OPERATIONS AND MANAGEMENT AND FOR PERSONS WORKING WITH OR HANDLING THIS PRODUCT. OLIN BELIEVES THIS INFORMATIONTO BE RELIABLE AND UPTO DATE AS OF THE DATE OF PUBLICATION, BUT MAKES NO WARRANTY THAT IT IS. ADDITIONALLY, IF AN SDS IS MORE THAN THREE YEARS OLD, YOU SHOULD CONTACT OLIN AT THE PHONE NUMBER BELOW TO MAKE CERTAIN THAT THE SDS IS CURRENT. SDS Control Group Olin Brass 305 Lewis and Clark Blvd East Alton, IL 62024-1197 Phone Number: (618) 258-5654 www.olinbrass.com Olin Brass SDS No.:01334.0001 Tin Coated Phosphor Bronze Revision Date: 6/1/15 Review Date: 1/1/17 Page 1 of 16 Safety Data Sheet 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: PHOSPHOR BRONZE ALLOYS Chemical Name: Metal Alloy Synonyms: Copper Tin Phosphorus Alloys; UNS/CDA Alloy Nos. c50000 – c52999 Chemical Family: Copper Formula: Not applicable - mixture Product Use: Metallurgical Products Manufacturer: SDS Control Group Technical Information: Emergency Information: Olin Brass (618)258-5654 (618)258-5167 305 Lewis and Clark Blvd East Alton, IL 62024-1197 www.olinbrass.com 2. -

Boosey & Hawkes
City Research Online City, University of London Institutional Repository Citation: Howell, Jocelyn (2016). Boosey & Hawkes: The rise and fall of a wind instrument manufacturing empire. (Unpublished Doctoral thesis, City, University of London) This is the accepted version of the paper. This version of the publication may differ from the final published version. Permanent repository link: https://openaccess.city.ac.uk/id/eprint/16081/ Link to published version: Copyright: City Research Online aims to make research outputs of City, University of London available to a wider audience. Copyright and Moral Rights remain with the author(s) and/or copyright holders. URLs from City Research Online may be freely distributed and linked to. Reuse: Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. City Research Online: http://openaccess.city.ac.uk/ [email protected] Boosey & Hawkes: The Rise and Fall of a Wind Instrument Manufacturing Empire Jocelyn Howell PhD in Music City University London, Department of Music July 2016 Volume 1 of 2 1 Table of Contents Table of Contents .................................................................................................................................... 2 Table of Figures...................................................................................................................................... -

Organ Pipes Differences in the Two Constructions
Reed Pipes Pipe Repair Reed Pipes are built from spotted metal (50% tin). Resonators longer than 2’ C on The OSI pipe shop is equipped to repair individual damaged metal flue, wood or reed Trumpets and Oboes are traditionally of two piece construction with spotted metal bells pipes or to provide faithful replacement pipes for those missing from an incomplete set. and zinc stems. Full length spotted metal resonators can be provided for 8’ and ½L 16’ When a pipe is to be repaired or replaced, the pipe on either side of the damaged or Reeds. Full length 16’ octaves are made from zinc with spotted metal bells. Common missing pipe is essential, along with operating wind pressure, so that a proper match for metal or antimonial lead can be used in place of spotted metal. both scaling and voicing can be obtained. Resonator Length Pipe Revoicing Full length reed resonators in the 16’ and 8’ octaves are always preferable for complete Older metal flue, wood or reed pipes in good physical condition can often be recycled development of sound, tonal weight and color. When a full length or mitered full length and revoiced at a savings. Pipes will be accepted for revoicing provided pipes are of bass is not practical, ½L resonators are an option for brighter chorus reeds such as good manufacture, show no metal fatigue and are not badly torn or mishandled. Pipes Trumpet, Trompette, Posaune, Fagotto and Oboe stops. ½L bases are not are washed and minor repairs are made. New slide tuners are installed, reed tuning recommended for predominantly dark or full bodied reeds in the Trombone, Tromba or wires and tongues are replaced in keeping with the new tonal requirements, wind Tuba class. -

Copper Alloys
THE COPPER ADVANTAGE A Guide to Working With Copper and Copper Alloys www.antimicrobialcopper.com CONTENTS I. Introduction ............................. 3 PREFACE Conductivity .....................................4 Strength ..........................................4 The information in this guide includes an overview of the well- Formability ......................................4 known physical, mechanical and chemical properties of copper, Joining ...........................................4 as well as more recent scientific findings that show copper has Corrosion ........................................4 an intrinsic antimicrobial property. Working and finishing Copper is Antimicrobial ....................... 4 techniques, alloy families, coloration and other attributes are addressed, illustrating that copper and its alloys are so Color ..............................................5 adaptable that they can be used in a multitude of applications Copper Alloy Families .......................... 5 in almost every industry, from door handles to electrical circuitry to heat exchangers. II. Physical Properties ..................... 8 Copper’s malleability, machinability and conductivity have Properties ....................................... 8 made it a longtime favorite metal of manufacturers and Electrical & Thermal Conductivity ........... 8 engineers, but it is its antimicrobial property that will extend that popularity into the future. This guide describes that property and illustrates how it can benefit everything from III. Mechanical -

C 544 Phosphor Bronze – Grade B Color Bronze Family: Leaded Phosphor Bronze Code
544 C 544 Phosphor Bronze – Grade B Color Bronze Family: Leaded Phosphor Bronze Code C 544 Phosphor Bronze – Grade B is a Morgan standard alloy and finds use in electrical applications, as well as many industrial applications. It is used as electrical connectors and partially due to its excellent machinability finds use as bushings, gears, pinions, screw machine products, thrust washers, valve parts, and shafts. Available from stock Equivalent Specifications at Morgan Bronze in: ASTM B139/ B139M Rounds Reference Specifications SAE J461; SAE J463 ASTM B103 Copper Alloy UNS C54400 Equivalent specifications are verified and updated annually. Specifications shown are current as of May 4, 2010. Chemical Composition (%) Cu Sn P Fe Pb Zn Remainder 3.5 – 4.5 0.01 – 0.50 0.10 max. 3.0 - 4.0 1.5 – 4.5 Sum of all named elements = 99.5% Mechanical Properties Minimum Tensile Properties, English/Metric Temper HO4 hard Nominal Diameter Tensile Strength Elongation** Inches/mm ksi MPa % ¼”– ½” incl./6 - 12 mm. incl. 60 415 10 Over ½” to 1” incl./12-25 mm incl. 55 380 12 Over 1.00”/25 mm 50 345 15 **Elongation in 4D Chemical Composition and Mechanical Properties shown pertain to ASTM B139/B139 M only. Machinability Rating 80 (Free Cutting Brass = 100) Phone: 847-526-6000 Toll Free: 800-445-9970 Fax: 847-438-6600 Email: [email protected] 544 Color C 544 Phosphor Bronze – Grade B Code Bronze Family: Phosphor Bronze (continued) Physical Properties English Metric Melting Point – Liquidus 1830° F 999° C Melting Point – Solidus 1700° F 927° C Density 0.320 -

What Hasn't Changed
presented by Steven Clagett Director, Nuclear and Missile Technology Controls Division Department of Commerce What Hasn’t Changed Dual use export controls do not pose a significant barrier to civil nuclear trade throughout the world. Jurisdiction is usually well defined. Disputes are usually handled informally, many times with only a phone call or e-mail. 1 Agency Jurisdiction Process Agency Jurisdiction Advisory Agencies Part 810 Department of Nuclear technology Departments of Energy and services related Commerce, to the production of Defense, State, and Special Nuclear the NRC Material Part 110 Nuclear Regulatory Nuclear equipment Departments of Commission (NRC) and material Commerce, Defense, State, and Energy Export Department of Dual-use items Departments of Administration Commerce listed on the Defense, Energy, Regulations Commerce Control and State List Commerce Jurisdiction (NSG dual-use items) Pressure Transducers (2B230) – Used for pressure measurement in a multitude of industrial processes as well as in the uranium hexafluoride gas in the centrifuge process for isotope separation Mass Spectrometers (3A233) – Determine composition of chemical samples as well as the chemical make-up of uranium, plutonium, etc. Machine Tools (2B001) – Product manufacturing in virtually every industry including nuclear explosive device components and uranium enrichment equipment 4 2 Commerce Jurisdiction (nuclear power related items) • Balance of plant – turbines, generators, switching gear, pipes and valves • Health and safety equipment – radiation -

Fretted Instrument Strings Introduction Index
Fretted Instrument Strings Introduction Index With the tremendous Electric Guitar Strings “Every song I’ve ever recorded has been variety of strings XLS Stainless Steel Round Wound 6 with D’Addario strings. The y’ve never available, choosing Pedal Steel 6 the right set for your XL Nickelplated Steel Round Wound 7 let me down - the best in the biz.” playing style and Heat-Treated Stainless Steel Half Round Wound 9 taste can be quite a Chromes Stainless Steel Flat Wound 9 confusing task, but it can also be a Acoustic Guitar Strings John 5 rewarding one. This 80/20 Bronze Round Wound 12 catalog has been Phosphor Bronze Round Wound 12 designed to provide Flat Tops Semi-Flattened Phosphor Bronze 13 you with all the Silk & Steel 13 technical information EXP Coated Phosphor Bronze Round Wound 14 you need to make EXP Coated 80/20 Bronze Round Wound 15 selecting your “D’Addario is tough! I’m never favorite strings as Bass Guitar Strings switching string brands!” easy as possible. It ProSteels Round Wound 18 also includes some XL Nickelplated Steel Round Wound 19 string tips to help EXP Coated Nickelplated Steel Round Wound 21 you get the most out Heat-Treated Nickel Half Round Wound 22 Herman Li of the strings you Chromes Stainless Steel Flat Wound 23 Dragonforce choose. We’re sure Phosphor Bronze Wound 23 you’ll find it useful. EXP Coated Phosphor Bronze Wound 23 Classical Guitar Strings Pro•Arté – Laser Selected Trebles 26 Pro•Arté Composites 26 EXP Coated – Laser Selected Trebles 27 Classics – Rectified Nylon Trebles 27 Classical Guitar – Student -

MUTES by You Do BESSON That 'DEAL' Better If You with Th·E Deal
PER AA ANNUAL SU3SCRIPTION REGISTERED FOR LIVERPOOL, FEBRU ARY 1, 1946 PRICE POST�· • No. 773 TRANSMISSION ABROAD 3d. Post Free 4/- QUARTETS POPULAR CORNET SOLOS BESSON FOR BRASS INSTRUMENTS NOW AVAILABLE (With Pianoforte Accompaniment) WILL BU Y YOUR ACCESSORIES s. d. 15 3 SURPLU S INSTRUMENTS Complete with Score and Parts New Standard Cornet Mouthpiece ... ... ... ... of 3 5 3 H. Moss "Mosquito" Trombone Valve Springs for Cornet, " N.V. A." ... per set 2 CORNETS 2 TROMBONES Valve Springs made of phosphor bronze- H. Moss " The Joker " Send particulars to : The Phantom Brigade . .. 2 6 3 6 Trumpets-Cornets-Horns ... ... ... set of I Demande et Reponse .. .. 2 6 Sutton " The Joy Wheel " BESSON, Department 19, West Street Baritone and Euphonium, 3 valve . ... ... 2 3 valve . .. 2 9 Clough "Winning Spurt" Charing 4 CORNETS Euphonium, 4 Cross Road, LONDON, W.C. 2 .. ... .. 2 9 Bal Masque 2 6 Eb and BBb Bass, 3 valves . .. Hartman "Facilita " Cornet . .. .. 3 3 Eb and BBb Bass, 4 valves .. .. TROMBONES 6 6 Percy Code "Lucille " 4 Waterkey Springs for all of above ... ... ... doz. Awake, Aeolian Lyre inside and outside, · · · · · above, complete sets "Zanette" · 2 o Corks for all of Percy Code Band Teachers, Adjudicators and Soloists Comrades in Arms } cork ... ... .. ... ... 2 6 including waterkey "Zelda" The Box of Soldiers ... ... 2 6 ... doz. 2 9 Percy Code Corks for waterkey, shellaced ready for fixing Baby's Sweetheart .. ... ... 2 6 11 "Triple Trumpeter" WILLIAM POLLAR Corks for waterkey, plain ... ... ... ... ... doz. I Grant D ... ... ... ... 10 3 2 CORNETS, HORN and EUPHONIUM Shanks for Cornet, Bb, silver-plated Templeton " Sonia" CORNET SOLOIST, BAND TEACHER, bottle I 6 Valve Oil .. -

Copper Alloys Form an Important Group of Metals with Many Excellent Properties
Helping manufacturers across the globe achieve sustainable leaner manufacturing processes Copper CUT TO Alloys LENGTH WIRE & COIL Commercially Pure High Conductivity Coppers Brasses Phosphor Bronzes Fast Nickel Silvers Turnaround Cupronickels Processing Beryllium Copper Pfinodal® Alloys Low Width Thickness Ratio 3:1 unique to the industry (normal minimum is 8:1) WIDE STOCK RANGE Over 75 years Experience FM 02114 Copper,Stainless Brasses Steel & Bronzes Copper and Copper alloys form an important group of metals with many excellent properties. They have very good electrical and thermal conductivities, are easy to fabricate and include some alloys of exceptional strength (notably Copper Beryllium Alloys) and corrosion resistance. The characteristics of Copper and Copper alloys have resulted in extensive use of those alloys in a very wide range of applications. They can be formed, pressed, deep drawn or photochemically etched into the most complex of shapes. COPPER, BRASS & BRONZE STOCK RANGE COIL STOCK RANGE WIRE STOCK RANGE Thickness (mm) Width (mm) Round Shaped COMMERCIALLY PURE HIGH CONDUCTIVITY COPPER 0.01 - 3.0 3 - 1220 0.1 – 10.00 mm dia upto 45 mm2 area BRASS 0.01 - 3.0 3 - 1220 0.1 – 10.00 mm dia upto 45 mm2 area PHOSPHOR BRASS 0.01 - 3.0 3 - 1220 0.1 – 10.00 mm dia upto 45 mm2 area NICKEL SILVERS 0.01 - 3.0 3 - 1220 0.1 – 10.00 mm dia upto 45 mm2 area CUPRONICKEL & HIGH COPPER CONTENT ALLOYS 0.01 - 3.0 3 - 1220 0.1 – 10.00 mm dia upto 45 mm2 area COPPER BERYLLIUM ALLOYS 0.01 - 3.0 3 - 1220 0.1 – 10.00 mm dia upto 45 mm2 area Other specifications can be made available upon request, please contact us with your requirements Types Commercially The various Coppers within this group have differing degrees of purity and consequently exhibit Pure High different characteristics. -
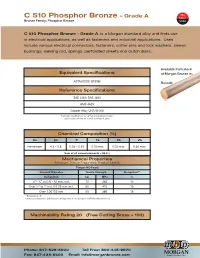
C51000 Phosphor Bronze
510 C 510 Phosphor Bronze – Grade A Color Bronze Family: Phosphor Bronze Code C 510 Phosphor Bronze – Grade A is a Morgan standard alloy and finds use in electrical applications, as well as fasteners and industrial applications. Uses include various electrical connectors, fasteners, cotter pins and lock washers, sleeve bushings, welding rod, springs, perforated sheets and clutch disks. Available from stock Equivalent Specifications at Morgan Bronze in: ASTM B139/ B139M Rounds Reference Specifications SAE J461; SAE J463 AMS 4625 Copper Alloy UNS 51000 Equivalent specifications are verified and updated annually. Specifications shown are current as of May 4, 2010. Chemical Composition (%) Cu Sn P Fe Pb Zn Remainder 4.2 – 5.8 0.03 – 0.35 0.10 max. 0.05 max. 0.30 max. Sum of all named elements = 99.5% Mechanical Properties Minimum Tensile Properties, English/Metric Temper HO4 hard Nominal Diameter Tensile Strength Elongation** Inches/mm ksi MPa % ¼”– ½” incl./6 - 12 mm. incl. 70 485 13 Over ½” to 1” incl./12-25 mm incl. 60 415 15 Over 1.00”/25 mm 55 380 18 **Elongation in 4D Chemical Composition and Mechanical Properties shown pertain to ASTM B139/B139 M only. Machinability Rating 20 (Free Cutting Brass = 100) Phone: 847-526-6000 Toll Free: 800-445-9970 Fax: 847-438-6600 Email: [email protected] 510 Color C 510 Phosphor Bronze – Grade A Code Bronze Family: Phosphor Bronze (continued) Physical Properties English Metric Melting Point – Liquidus 1920° F 1049° C Melting Point – Solidus 1750° F 954° C Density 0.320 lb/in³ at 68° F 8.86 gm/cm³ -

Dayton C. Miller Flute Collection
Guides to Special Collections in the Music Division at the Library of Congress Dayton C. Miller Flute Collection LIBRARY OF CONGRESS WASHINGTON 2004 Table of Contents Introduction ......................................................................................................................................................... iii Biographical Sketch.............................................................................................................................................. vi Scope and Content Note..................................................................................................................................... viii Description of Series............................................................................................................................................. xi Container List ........................................................................................................................................................ 1 FLUTES OF DAYTON C. MILLER............................................................................................................... 1 ii Introduction Thomas Jefferson's library is the foundation of the collections of the Library of Congress. Congress purchased it to replace the books that had been destroyed in 1814, when the Capitol was burned during the War of 1812. Reflecting Jefferson's universal interests and knowledge, the acquisition established the broad scope of the Library's future collections, which, over the years, were enriched by copyright -

A Complete Technical Reference for Fretted Instrument String Tensions Table of Contents Introduction
Catalog Supplement/String Tension Specifications A complete technical reference for fretted instrument string tensions Table of Contents Introduction Technical Reference for fretted instrument string tensions 4 his brochure was created to assist in Guitar and Bass Fingerboard Legends 5 Tselecting the ideal string for your instrument and playing style. Included in this brochure String Tensions are tension tables and formulas to help you Acoustic or Electric Guitar 6 calculate what tension a particular string will Pedal Steel Guitar 7 have on your instrument. Acoustic Guitar 7 Classical Guitar 8 Folk Guitar 10 Electric Bass Guitar 10 Acoustic Bass Guitar 13 Mandolin Family Strings 13 Banjo & Loop End Strings 14 A complete technical reference for fretted instrument string tensions Understanding what determines string tension. In order to determine the tension at which a string will vibrate, you need three pieces of information: the Unit Weight, the Scale Length, and the Frequency of the string. You can use the charts in this brochure to get a pre-calculated tension for the D’Addario strings listed or you can use the formulas below to calculate the exact tension for any string using the scale length of your particular instrument. All of the charts illustrate string tensions for each string at a variety of pitches, in case you use alternative tunings. UW- Unit Weight. In all the charts and formulas in the brochure, unit weight is expressed in pounds per linear inch (lb/in). L- Scale Length. This is the vibrating length of the string. This is determined by measuring the distance from the nut to the bridge of the instrument in inches (in).