Download This PDF File
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Natural History of Reproduction in Solanum and Lycianthes (Solanaceae) in a Subtropical Moist Forest
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications in the Biological Sciences Papers in the Biological Sciences 11-28-2002 The Natural History of Reproduction in Solanum and Lycianthes (Solanaceae) in a Subtropical Moist Forest Stacey DeWitt Smith University of Nebraska - Lincoln, [email protected] Sandra Knapp Natural History Museum, London Follow this and additional works at: https://digitalcommons.unl.edu/bioscifacpub Part of the Life Sciences Commons Smith, Stacey DeWitt and Knapp, Sandra, "The Natural History of Reproduction in Solanum and Lycianthes (Solanaceae) in a Subtropical Moist Forest" (2002). Faculty Publications in the Biological Sciences. 104. https://digitalcommons.unl.edu/bioscifacpub/104 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications in the Biological Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Bull. nat. Hist. Mus. Lond. (Bot.) 32(2): 125–136 Issued 28 November 2002 The natural history of reproduction in Solanum and Lycianthes (Solanaceae) in a subtropical moist forest STACEY D. SMITH Department of Botany, 132 Birge Hall, 430 Lincoln Drive, University of Wisconsin, Madison WI 53706-1381, U.S.A. SANDRA KNAPP Department of Botany, The Natural History Museum, Cromwell Road, London SW7 5BD CONTENTS Introduction ............................................................................................................................................................................ -

Chec List What Survived from the PLANAFLORO Project
Check List 10(1): 33–45, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution What survived from the PLANAFLORO Project: PECIES S Angiosperms of Rondônia State, Brazil OF 1* 2 ISTS L Samuel1 UniCarleialversity of Konstanz, and Narcísio Department C.of Biology, Bigio M842, PLZ 78457, Konstanz, Germany. [email protected] 2 Universidade Federal de Rondônia, Campus José Ribeiro Filho, BR 364, Km 9.5, CEP 76801-059. Porto Velho, RO, Brasil. * Corresponding author. E-mail: Abstract: The Rondônia Natural Resources Management Project (PLANAFLORO) was a strategic program developed in partnership between the Brazilian Government and The World Bank in 1992, with the purpose of stimulating the sustainable development and protection of the Amazon in the state of Rondônia. More than a decade after the PLANAFORO program concluded, the aim of the present work is to recover and share the information from the long-abandoned plant collections made during the project’s ecological-economic zoning phase. Most of the material analyzed was sterile, but the fertile voucher specimens recovered are listed here. The material examined represents 378 species in 234 genera and 76 families of angiosperms. Some 8 genera, 68 species, 3 subspecies and 1 variety are new records for Rondônia State. It is our intention that this information will stimulate future studies and contribute to a better understanding and more effective conservation of the plant diversity in the southwestern Amazon of Brazil. Introduction The PLANAFLORO Project funded botanical expeditions In early 1990, Brazilian Amazon was facing remarkably in different areas of the state to inventory arboreal plants high rates of forest conversion (Laurance et al. -

Two New Species of Lycianthes Series Tricolores from Eastern Mexico
Dean, E.A. 2014. Two new species of Lycianthes series Tricolores from eastern Mexico. Phytoneuron 2014-42: 1–6. Published 2 April 2014. ISSN 2153 733X TWO NEW SPECIES OF LYCIANTHES SERIES TRICOLORES FROM EASTERN MEXICO ELLEN A. DEAN UC Davis Center for Plant Diversity Plant Sciences M.S. 7 One Shields Avenue Davis, California 95616 [email protected] ABSTRACT Two new species, Lycianthes venturana E. Dean, sp. nov. and Lycianthes michaelneei E. Dean, sp. nov. , are described from eastern Mexico, the former from the state of Puebla and the latter from the state of Veracruz. Both species belong to series Tricolores Bitter, which is made up of six species of shrubs with simple trichomes and that are distributed from Nicaragua to western Mexico. Lycianthes (Dun.) Hassler (Solanaceae) has both Old World and New World representatives and includes 150 to 200 species (Hunziker 2001). Its center of distribution, and the majority of its taxa, are found in the New World (from Mexico to Argentina), with ca. 30 taxa native to Mexico (ca. 14 endemic, two of them described here). The genus is the closest relative of the chile pepper genus Capsicum L. (Walsh & Hoot 2001; Bohs & Olmstead 1997), a relationship that was first pointed out by the German botanist Georg Bitter (Bitter 1919). The two genera have a similar calyx morphology, with the five lobes of the calyx truncated into a sleeve, below which may protrude five to ten appendages (commonly called calyx teeth). However, while Capsicum species have anthers that dehisce by longitudinal slits, the species of Lycianthes typically have poricidal anther dehiscence. -

Chorological Notes on the Non-Native Flora of the Province of Tarragona (Catalonia, Spain)
Butlletí de la Institució Catalana d’Història Natural, 83: 133-146. 2019 ISSN 2013-3987 (online edition): ISSN: 1133-6889 (print edition)133 GEA, FLORA ET fauna GEA, FLORA ET FAUNA Chorological notes on the non-native flora of the province of Tarragona (Catalonia, Spain) Filip Verloove*, Pere Aymerich**, Carlos Gómez-Bellver*** & Jordi López-Pujol**** * Meise Botanic Garden, Nieuwelaan 38, B-1860 Meise, Belgium. ** C/ Barcelona 29, 08600 Berga, Barcelona, Spain. *** Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals. Secció Botànica i Micologia. Facultat de Biologia. Universitat de Barcelona. Avda. Diagonal, 643. 08028 Barcelona, Spain. **** Botanic Institute of Barcelona (IBB, CSIC-ICUB). Passeig del Migdia. 08038 Barcelona, Spain. Author for correspondence: F. Verloove. A/e: [email protected] Rebut: 10.07.2019; Acceptat: 16.07.2019; Publicat: 30.09.2019 Abstract Recent field work in the province of Tarragona (NE Spain, Catalonia) yielded several new records of non-native vascular plants. Cenchrus orientalis, Manihot grahamii, Melica chilensis and Panicum capillare subsp. hillmanii are probably reported for the first time from Spain, while Aloe ferox, Canna ×generalis, Cenchrus setaceus, Convolvulus farinosus, Ficus rubiginosa, Jarava plumosa, Koelreu- teria paniculata, Lycianthes rantonnetii, Nassella tenuissima, Paraserianthes lophantha, Plumbago auriculata, Podranea ricasoliana, Proboscidea louisianica, Sedum palmeri, Solanum bonariense, Tipuana tipu, Tradescantia pallida and Vitis ×ruggerii are reported for the first time from the province of Tarragona. Several of these are potential or genuine invasive species and/or agricultural weeds. Miscellane- ous additional records are presented for some further alien taxa with only few earlier records in the study area. Key words: Alien plants, Catalonia, chorology, Spain, Tarragona, vascular plants. -

Illustrated Flora of East Texas Illustrated Flora of East Texas
ILLUSTRATED FLORA OF EAST TEXAS ILLUSTRATED FLORA OF EAST TEXAS IS PUBLISHED WITH THE SUPPORT OF: MAJOR BENEFACTORS: DAVID GIBSON AND WILL CRENSHAW DISCOVERY FUND U.S. FISH AND WILDLIFE FOUNDATION (NATIONAL PARK SERVICE, USDA FOREST SERVICE) TEXAS PARKS AND WILDLIFE DEPARTMENT SCOTT AND STUART GENTLING BENEFACTORS: NEW DOROTHEA L. LEONHARDT FOUNDATION (ANDREA C. HARKINS) TEMPLE-INLAND FOUNDATION SUMMERLEE FOUNDATION AMON G. CARTER FOUNDATION ROBERT J. O’KENNON PEG & BEN KEITH DORA & GORDON SYLVESTER DAVID & SUE NIVENS NATIVE PLANT SOCIETY OF TEXAS DAVID & MARGARET BAMBERGER GORDON MAY & KAREN WILLIAMSON JACOB & TERESE HERSHEY FOUNDATION INSTITUTIONAL SUPPORT: AUSTIN COLLEGE BOTANICAL RESEARCH INSTITUTE OF TEXAS SID RICHARDSON CAREER DEVELOPMENT FUND OF AUSTIN COLLEGE II OTHER CONTRIBUTORS: ALLDREDGE, LINDA & JACK HOLLEMAN, W.B. PETRUS, ELAINE J. BATTERBAE, SUSAN ROBERTS HOLT, JEAN & DUNCAN PRITCHETT, MARY H. BECK, NELL HUBER, MARY MAUD PRICE, DIANE BECKELMAN, SARA HUDSON, JIM & YONIE PRUESS, WARREN W. BENDER, LYNNE HULTMARK, GORDON & SARAH ROACH, ELIZABETH M. & ALLEN BIBB, NATHAN & BETTIE HUSTON, MELIA ROEBUCK, RICK & VICKI BOSWORTH, TONY JACOBS, BONNIE & LOUIS ROGNLIE, GLORIA & ERIC BOTTONE, LAURA BURKS JAMES, ROI & DEANNA ROUSH, LUCY BROWN, LARRY E. JEFFORDS, RUSSELL M. ROWE, BRIAN BRUSER, III, MR. & MRS. HENRY JOHN, SUE & PHIL ROZELL, JIMMY BURT, HELEN W. JONES, MARY LOU SANDLIN, MIKE CAMPBELL, KATHERINE & CHARLES KAHLE, GAIL SANDLIN, MR. & MRS. WILLIAM CARR, WILLIAM R. KARGES, JOANN SATTERWHITE, BEN CLARY, KAREN KEITH, ELIZABETH & ERIC SCHOENFELD, CARL COCHRAN, JOYCE LANEY, ELEANOR W. SCHULTZE, BETTY DAHLBERG, WALTER G. LAUGHLIN, DR. JAMES E. SCHULZE, PETER & HELEN DALLAS CHAPTER-NPSOT LECHE, BEVERLY SENNHAUSER, KELLY S. DAMEWOOD, LOGAN & ELEANOR LEWIS, PATRICIA SERLING, STEVEN DAMUTH, STEVEN LIGGIO, JOE SHANNON, LEILA HOUSEMAN DAVIS, ELLEN D. -

Un-Priced 2021 Catalog in PDF Format
c toll free: 800.438.7199 fax: 805.964.1329 local: 805.683.1561 text: 805.243.2611 acebook.com/SanMarcosGrowers email: [email protected] Our world certainly has changed since we celebrated 40 years in business with our October 2019 Field Day. Who knew then that we were only months away from a global pandemic that would disrupt everything we thought of as normal, and that the ensuing shutdown would cause such increased interest in gardening? This past year has been a rollercoaster ride for all of us in the nursery and landscape trades. The demand for plants so exceeded the supply that it caused major plant availability shortages, and then the freeze in Texas further exacerbated this situation. To ensure that our customers came first, we did not sell any plants out-of-state, and we continue to work hard to refill the empty spaces left in our field. In the chaos of the situation, we also decided not to produce a 2020 catalog, and this current catalog is coming out so late that we intend it to be a two-year edition. Some items listed may not be available until early next year, so we encourage customers to look to our website Primelist which is updated weekly to view our current availability. As in the past, we continue to grow the many tried-and-true favorite plants that have proven themselves in our warming mediterranean climate. We have also added 245 exciting new plants that are listed in the back of this catalog. With sincere appreciation to all our customers, it is our hope that 2021 and 2022 will be excellent years for horticulture! In House Sales Outside Sales Shipping Ethan Visconti - Ext 129 Matthew Roberts Michael Craib Gene Leisch - Ext 128 Sales Manager Sales Representative Sales Representative Shipping Manager - Vice President [email protected] (805) 452-7003 (805) 451-0876 [email protected] [email protected] [email protected] Roger Barron- Ext 126 Jose Bedolla Sales/ Customer Service Serving nurseries in: Serving nurseries in: John Dudley, Jr. -

A Molecular Phylogeny of the Solanaceae
TAXON 57 (4) • November 2008: 1159–1181 Olmstead & al. • Molecular phylogeny of Solanaceae MOLECULAR PHYLOGENETICS A molecular phylogeny of the Solanaceae Richard G. Olmstead1*, Lynn Bohs2, Hala Abdel Migid1,3, Eugenio Santiago-Valentin1,4, Vicente F. Garcia1,5 & Sarah M. Collier1,6 1 Department of Biology, University of Washington, Seattle, Washington 98195, U.S.A. *olmstead@ u.washington.edu (author for correspondence) 2 Department of Biology, University of Utah, Salt Lake City, Utah 84112, U.S.A. 3 Present address: Botany Department, Faculty of Science, Mansoura University, Mansoura, Egypt 4 Present address: Jardin Botanico de Puerto Rico, Universidad de Puerto Rico, Apartado Postal 364984, San Juan 00936, Puerto Rico 5 Present address: Department of Integrative Biology, 3060 Valley Life Sciences Building, University of California, Berkeley, California 94720, U.S.A. 6 Present address: Department of Plant Breeding and Genetics, Cornell University, Ithaca, New York 14853, U.S.A. A phylogeny of Solanaceae is presented based on the chloroplast DNA regions ndhF and trnLF. With 89 genera and 190 species included, this represents a nearly comprehensive genus-level sampling and provides a framework phylogeny for the entire family that helps integrate many previously-published phylogenetic studies within So- lanaceae. The four genera comprising the family Goetzeaceae and the monotypic families Duckeodendraceae, Nolanaceae, and Sclerophylaceae, often recognized in traditional classifications, are shown to be included in Solanaceae. The current results corroborate previous studies that identify a monophyletic subfamily Solanoideae and the more inclusive “x = 12” clade, which includes Nicotiana and the Australian tribe Anthocercideae. These results also provide greater resolution among lineages within Solanoideae, confirming Jaltomata as sister to Solanum and identifying a clade comprised primarily of tribes Capsiceae (Capsicum and Lycianthes) and Physaleae. -

Evolutionary Routes to Biochemical Innovation Revealed by Integrative
RESEARCH ARTICLE Evolutionary routes to biochemical innovation revealed by integrative analysis of a plant-defense related specialized metabolic pathway Gaurav D Moghe1†, Bryan J Leong1,2, Steven M Hurney1,3, A Daniel Jones1,3, Robert L Last1,2* 1Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, United States; 2Department of Plant Biology, Michigan State University, East Lansing, United States; 3Department of Chemistry, Michigan State University, East Lansing, United States Abstract The diversity of life on Earth is a result of continual innovations in molecular networks influencing morphology and physiology. Plant specialized metabolism produces hundreds of thousands of compounds, offering striking examples of these innovations. To understand how this novelty is generated, we investigated the evolution of the Solanaceae family-specific, trichome- localized acylsugar biosynthetic pathway using a combination of mass spectrometry, RNA-seq, enzyme assays, RNAi and phylogenomics in different non-model species. Our results reveal hundreds of acylsugars produced across the Solanaceae family and even within a single plant, built on simple sugar cores. The relatively short biosynthetic pathway experienced repeated cycles of *For correspondence: [email protected] innovation over the last 100 million years that include gene duplication and divergence, gene loss, evolution of substrate preference and promiscuity. This study provides mechanistic insights into the † Present address: Section of emergence of plant chemical novelty, and offers a template for investigating the ~300,000 non- Plant Biology, School of model plant species that remain underexplored. Integrative Plant Sciences, DOI: https://doi.org/10.7554/eLife.28468.001 Cornell University, Ithaca, United States Competing interests: The authors declare that no Introduction competing interests exist. -

SOLANACEAE 茄科 Qie Ke Zhang Zhi-Yun, Lu An-Ming; William G
Flora of China 17: 300–332. 1994. SOLANACEAE 茄科 qie ke Zhang Zhi-yun, Lu An-ming; William G. D'Arcy Herbs, shrubs, small trees, or climbers. Stems sometimes prickly, rarely thorny; hairs simple, branched, or stellate, sometimes glandular. Leaves alternate, solitary or paired, simple or pinnately compound, without stipules; leaf blade entire, dentate, lobed, or divided. Inflorescences terminal, overtopped by continuing axes, appearing axillary, extra-axillary, or leaf opposed, often apparently umbellate, racemose, paniculate, clustered, or solitary flowers, rarely true cymes, sometimes bracteate. Flowers mostly bisexual, usually regular, 5-merous, rarely 4- or 6–9-merous. Calyx mostly lobed. Petals united. Stamens as many as corolla lobes and alternate with them, inserted within corolla, all alike or 1 or more reduced; anthers dehiscing longitudinally or by apical pores. Ovary 2–5-locular; placentation mostly axile; ovules usually numerous. Style 1. Fruiting calyx often becoming enlarged, mostly persistent. Fruit a berry or capsule. Seeds with copious endosperm; embryo mostly curved. About 95 genera with 2300 species: best represented in western tropical America, widespread in temperate and tropical regions; 20 genera (ten introduced) and 101 species in China. Some species of Solanaceae are known in China only by plants cultivated in ornamental or specialty gardens: Atropa belladonna Linnaeus, Cyphomandra betacea (Cavanilles) Sendtner, Brugmansia suaveolens (Willdenow) Berchtold & Presl, Nicotiana alata Link & Otto, and Solanum jasminoides Paxton. Kuang Ko-zen & Lu An-ming, eds. 1978. Solanaceae. Fl. Reipubl. Popularis Sin. 67(1): 1–175. 1a. Flowers in several- to many-flowered inflorescences; peduncle mostly present and evident. 2a. Fruit enclosed in fruiting calyx. -

Scientific Opinion on the Assessment of the Risk of Solanaceous Pospiviroids for the EU Territory and the Identification and Evaluation of Risk Management Options1
EFSA Journal 2011;9(8):2330 SCIENTIFIC OPINION Scientific Opinion on the assessment of the risk of solanaceous pospiviroids for the EU territory and the identification and evaluation of risk 1 management options EFSA Panel on Plant Health (PLH)2, 3 European Food Safety Authority (EFSA), Parma, Italy ABSTRACT Following a request from the EU Commission, the EFSA PLH Panel conducted a risk assessment for the EU territory of pospiviroids affecting solanaceous crops, identified and evaluated risk reduction options and evaluated the EU provisional emergency measures targeting Potato spindle tuber viroid (PSTVd). The risk assessment included PSTVd, Citrus exocortis viroid, Columnea latent viroid, Mexican papita viroid, Tomato apical stunt viroid, Tomato chlorotic dwarf viroid, Tomato planta macho viroid, Chrysanthemum stunt viroid and Pepper chat fruit viroid. Four entry pathways were identified, three involving plant propagation material, with moderate probability of entry, and one involving plant products for human consumption, with low probability of entry. The probability of establishment was considered very high. Spread was considered likely within a crop and moderately likely between crop species, with exception of spread to potato, rated as unlikely. The probability of long distance spread within vegetatively propagated crops was estimated as likely/very likely. The direct consequences were expected to be major in potato and tomato, moderate in pepper, minimal/minor in other vegetables and minimal in ornamentals. Main risk assessment uncertainties derive from limited knowledge on pospiviroids other than PSTVd, although all pospiviroids are expected to have similar biological properties. Management options to reduce risk of entry, spread and consequences were identified and evaluated. -

Downloaded from the National Center for Biotechnology Information Database
J. AMER.SOC.HORT.SCI. 144(5):363–374. 2019. https://doi.org/10.21273/JASHS04735-19 DNA Barcoding of the Solanaceae Family in Puerto Rico Including Endangered and Endemic Species Lumariz Hernandez Rosario, Juan O. Rodríguez Padilla, Desiree Ramos Martínez, Alejandra Morales Grajales, Joel A. Mercado Reyes, Gabriel J. Veintidos Feliu, Benjamin Van Ee, and Dimuth Siritunga1 Department of Biology, University of Puerto Rico Mayaguez Campus, Mayaguez, PR 00680 ADDITIONAL INDEX WORDS. species identification, psbA-trnH, matK, ITS ABSTRACT. The Solanaceae family is one of the largest and well-distributed plant families in the world. It contains species of agricultural and economical importance, such as Solanum tuberosum, Solanum melongena, Solanum lycopersicum, Nicotiana tabacum, and Capsicum annuum. In Puerto Rico, there are ’46 species of Solanaceae of which six are endemic: Brunfelsia densifolia, Brunfelsia lactea, Brunfelsia portoricensis, Goetzea elegans, Solanum ensifolium, and Solanum woodburyi. Our objective was to use DNA barcoding to identify the Solanaceae species in Puerto Rico, including the endemics, and to assess the species relationships between them. To accomplish our objective, two chloroplast regions (psbA-trnH and matK) and a nuclear region [internal transcribed spacer (ITS)] were assessed. Pairwise distance and phylogenetic analysis demonstrate that DNA barcoding can be used to discriminate at the species level among these taxa in Puerto Rico. For all three markers, the genus that showed the highest pairwise distance between represented species was Solanum, whereas the genus that displayed the least was Capsicum. Phylogenetic trees of single and concatenated regions were generated from sequences obtained in this study and from data downloaded from the National Center for Biotechnology Information database. -
2007 Catalog
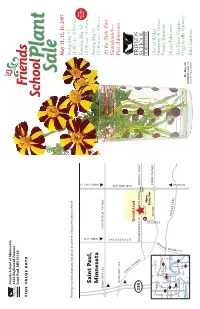
Friends School of Minnesota 1365 Englewood Avenue Saint Paul, MN 55104 TIME VALUE DATA May 11, 12, 13, 2007 Friday,May 11 If you have received a duplicate copy, please let us know, and pass the extra to a friend! 11:00 A.M.–8:00 P.M. New Saturday Saturday,May 12 Hours Saint Paul, 10:00 A.M.–6:00 P.M. Sunday,May 13 FROM 35W Minnesota FROM HWY 36 12:00 NOON–4:00 P.M. FROM HWY 280 LARPENTEUR AVENUE At the State Fair Grandstand— FROM HWY 280 Free Admission C O M O CLEVELAND AVE A SNELLING AVE V E Grandstand N U 280 E COMMONWEALTH DAN PATCH Main MIDWAY PKWY Gate P Minn. State Fair 94 Coliseum COMO AVENUE 35W White Shoreview Glacial Ridge Brooklyn Ctr Bear Lake 694 35E E U CANFIELD Growers: A Green Plymouth Crystal 94 Roseville N 36 E 494 Snelling Ave. 694 V 169 Saint Paul Family Business 280 A 394 35E 100 94 D Minnetonka Minneapolis E N N Woodbury ERGY Hosta Takeover! O P Edina 494 ARK 62 M Richfield Y 61 Eden 494 Prairie A Are These Veggies 35W Inver Grove R Heights Bloomington Eagan FROM 94 Organically Grown? 52 Mr. Majestic Shakopee 35E Burnsville marigold, page 12 Photo by Nancy Scherer Bird Gardens 18th Annual Friends School Plant Sale May 11, 12 and 13, 2007 Friday 11:00 A.M.–8:00 P.M.• Saturday 10:00 A.M.–6:00 P.M. Sunday 12:00 NOON–4:00 P.M.Sunday is half-price day at the Minnesota State Fair Grandstand Friends School of Minnesota Thank you for supporting Friends School of Minnesota by purchasing plants at our sale.