30.2 Pauli Paramagnetism
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Arxiv:Cond-Mat/0106646 29 Jun 2001 Landau Diamagnetism Revisited
Landau Diamagnetism Revisited Sushanta Dattagupta†,**, Arun M. Jayannavar‡ and Narendra Kumar# †S.N. Bose National Centre for Basic Sciences, Block JD, Sector III, Salt Lake, Kolkata 700 098, India ‡Institute of Physics, Sachivalaya Marg, Bhubaneswar 751 005, India #Raman Research Institute, Bangalore 560 080, India The problem of diamagnetism, solved by Landau, continues to pose fascinating issues which have relevance even today. These issues relate to inherent quantum nature of the problem, the role of boundary and dissipation, the meaning of thermodynamic limits, and above all, the quantum–classical crossover occasioned by environment- induced decoherence. The Landau diamagnetism provides a unique paradigm for discussing these issues, the significance of which is far-reaching. Our central result connects the mean orbital magnetic moment, a thermodynamic property, with the electrical resistivity, which characterizes transport properties of material. In this communication, we wish to draw the attention of Peierls to term diamagnetism as one of the surprises in the reader to certain enigmatic issues concerning dia- theoretical physics4. Landau’s pathbreaking result also magnetism. Indeed, diamagnetism can be used as a demonstrated that the calculation of diamagnetic suscep- prototype phenomenon to illustrate the essential role of tibility did indeed require an explicit quantum treatment. quantum mechanics, surface–boundary, dissipation and Turning to the classical domain, two of the present nonequilibrium statistical mechanics itself. authors had worried, some years ago, about the issue: Diamagnetism is a material property that characterizes does the BV theorem survive dissipation5? This is a natu- the response of an ensemble of charged particles (more ral question to ask as dissipation is a ubiquitous property specifically, electrons) to an applied magnetic field. -

Lecture 3: Fermi-Liquid Theory 1 General Considerations Concerning Condensed Matter
Phys 769 Selected Topics in Condensed Matter Physics Summer 2010 Lecture 3: Fermi-liquid theory Lecturer: Anthony J. Leggett TA: Bill Coish 1 General considerations concerning condensed matter (NB: Ultracold atomic gasses need separate discussion) Assume for simplicity a single atomic species. Then we have a collection of N (typically 1023) nuclei (denoted α,β,...) and (usually) ZN electrons (denoted i,j,...) interacting ∼ via a Hamiltonian Hˆ . To a first approximation, Hˆ is the nonrelativistic limit of the full Dirac Hamiltonian, namely1 ~2 ~2 1 e2 1 Hˆ = 2 2 + NR −2m ∇i − 2M ∇α 2 4πǫ r r α 0 i j Xi X Xij | − | 1 (Ze)2 1 1 Ze2 1 + . (1) 2 4πǫ0 Rα Rβ − 2 4πǫ0 ri Rα Xαβ | − | Xiα | − | For an isolated atom, the relevant energy scale is the Rydberg (R) – Z2R. In addition, there are some relativistic effects which may need to be considered. Most important is the spin-orbit interaction: µ Hˆ = B σ (v V (r )) (2) SO − c2 i · i × ∇ i Xi (µB is the Bohr magneton, vi is the velocity, and V (ri) is the electrostatic potential at 2 3 2 ri as obtained from HˆNR). In an isolated atom this term is o(α R) for H and o(Z α R) for a heavy atom (inner-shell electrons) (produces fine structure). The (electron-electron) magnetic dipole interaction is of the same order as HˆSO. The (electron-nucleus) hyperfine interaction is down relative to Hˆ by a factor µ /µ 10−3, and the nuclear dipole-dipole SO n B ∼ interaction by a factor (µ /µ )2 10−6. -

Phys 446: Solid State Physics / Optical Properties Lattice Vibrations
Solid State Physics Lecture 5 Last week: Phys 446: (Ch. 3) • Phonons Solid State Physics / Optical Properties • Today: Einstein and Debye models for thermal capacity Lattice vibrations: Thermal conductivity Thermal, acoustic, and optical properties HW2 discussion Fall 2007 Lecture 5 Andrei Sirenko, NJIT 1 2 Material to be included in the test •Factors affecting the diffraction amplitude: Oct. 12th 2007 Atomic scattering factor (form factor): f = n(r)ei∆k⋅rl d 3r reflects distribution of electronic cloud. a ∫ r • Crystalline structures. 0 sin()∆k ⋅r In case of spherical distribution f = 4πr 2n(r) dr 7 crystal systems and 14 Bravais lattices a ∫ n 0 ∆k ⋅r • Crystallographic directions dhkl = 2 2 2 1 2 ⎛ h k l ⎞ 2πi(hu j +kv j +lw j ) and Miller indices ⎜ + + ⎟ •Structure factor F = f e ⎜ a2 b2 c2 ⎟ ∑ aj ⎝ ⎠ j • Definition of reciprocal lattice vectors: •Elastic stiffness and compliance. Strain and stress: definitions and relation between them in a linear regime (Hooke's law): σ ij = ∑Cijklε kl ε ij = ∑ Sijklσ kl • What is Brillouin zone kl kl 2 2 C •Elastic wave equation: ∂ u C ∂ u eff • Bragg formula: 2d·sinθ = mλ ; ∆k = G = eff x sound velocity v = ∂t 2 ρ ∂x2 ρ 3 4 • Lattice vibrations: acoustic and optical branches Summary of the Last Lecture In three-dimensional lattice with s atoms per unit cell there are Elastic properties – crystal is considered as continuous anisotropic 3s phonon branches: 3 acoustic, 3s - 3 optical medium • Phonon - the quantum of lattice vibration. Elastic stiffness and compliance tensors relate the strain and the Energy ħω; momentum ħq stress in a linear region (small displacements, harmonic potential) • Concept of the phonon density of states Hooke's law: σ ij = ∑Cijklε kl ε ij = ∑ Sijklσ kl • Einstein and Debye models for lattice heat capacity. -

Solid State Physics 2 Lecture 5: Electron Liquid
Physics 7450: Solid State Physics 2 Lecture 5: Electron liquid Leo Radzihovsky (Dated: 10 March, 2015) Abstract In these lectures, we will study itinerate electron liquid, namely metals. We will begin by re- viewing properties of noninteracting electron gas, developing its Greens functions, analyzing its thermodynamics, Pauli paramagnetism and Landau diamagnetism. We will recall how its thermo- dynamics is qualitatively distinct from that of a Boltzmann and Bose gases. As emphasized by Sommerfeld (1928), these qualitative di↵erence are due to the Pauli principle of electons’ fermionic statistics. We will then include e↵ects of Coulomb interaction, treating it in Hartree and Hartree- Fock approximation, computing the ground state energy and screening. We will then study itinerate Stoner ferromagnetism as well as various response functions, such as compressibility and conduc- tivity, and screening (Thomas-Fermi, Debye). We will then discuss Landau Fermi-liquid theory, which will allow us understand why despite strong electron-electron interactions, nevertheless much of the phenomenology of a Fermi gas extends to a Fermi liquid. We will conclude with discussion of electrons on the lattice, treated within the Hubbard and t-J models and will study transition to a Mott insulator and magnetism 1 I. INTRODUCTION A. Outline electron gas ground state and excitations • thermodynamics • Pauli paramagnetism • Landau diamagnetism • Hartree-Fock theory of interactions: ground state energy • Stoner ferromagnetic instability • response functions • Landau Fermi-liquid theory • electrons on the lattice: Hubbard and t-J models • Mott insulators and magnetism • B. Background In these lectures, we will study itinerate electron liquid, namely metals. In principle a fully quantum mechanical, strongly Coulomb-interacting description is required. -

Chapter 3 Bose-Einstein Condensation of an Ideal
Chapter 3 Bose-Einstein Condensation of An Ideal Gas An ideal gas consisting of non-interacting Bose particles is a ¯ctitious system since every realistic Bose gas shows some level of particle-particle interaction. Nevertheless, such a mathematical model provides the simplest example for the realization of Bose-Einstein condensation. This simple model, ¯rst studied by A. Einstein [1], correctly describes important basic properties of actual non-ideal (interacting) Bose gas. In particular, such basic concepts as BEC critical temperature Tc (or critical particle density nc), condensate fraction N0=N and the dimensionality issue will be obtained. 3.1 The ideal Bose gas in the canonical and grand canonical ensemble Suppose an ideal gas of non-interacting particles with ¯xed particle number N is trapped in a box with a volume V and at equilibrium temperature T . We assume a particle system somehow establishes an equilibrium temperature in spite of the absence of interaction. Such a system can be characterized by the thermodynamic partition function of canonical ensemble X Z = e¡¯ER ; (3.1) R where R stands for a macroscopic state of the gas and is uniquely speci¯ed by the occupa- tion number ni of each single particle state i: fn0; n1; ¢ ¢ ¢ ¢ ¢ ¢g. ¯ = 1=kBT is a temperature parameter. Then, the total energy of a macroscopic state R is given by only the kinetic energy: X ER = "ini; (3.2) i where "i is the eigen-energy of the single particle state i and the occupation number ni satis¯es the normalization condition X N = ni: (3.3) i 1 The probability -

1 Introduction 2 Spins As Quantized Magnetic Moments
Spin 1 Introduction For the past few weeks you have been learning about the mathematical and algorithmic background of quantum computation. Over the course of the next couple of lectures, we'll discuss the physics of making measurements and performing qubit operations in an actual system. In particular we consider nuclear magnetic resonance (NMR). Before we get there, though, we'll discuss a very famous experiment by Stern and Gerlach. 2 Spins as quantized magnetic moments The quantum two level system is, in many ways, the simplest quantum system that displays interesting behavior (this is a very subjective statement!). Before diving into the physics of two-level systems, a little on the history of the canonical example: electron spin. In 1921, Stern proposed an experiment to distinguish between Larmor's classical theory of the atom and Sommerfeld's quantum theory. Each theory predicted that the atom should have a magnetic moment (i.e., it should act like a small bar magnet). However, Larmor predicted that this magnetic moment could be oriented along any direction in space, while Sommerfeld (with help from Bohr) predicted that the orientation could only be in one of two directions (in this case, aligned or anti-aligned with a magnetic field). Stern's idea was to use the fact that magnetic moments experience a linear force when placed in a magnetic field gradient. To see this, not that the potential energy of a magnetic dipole in a magnetic field is given by: U = −~µ · B~ Here, ~µ is the vector indicating the magnitude and direction of the magnetic moment. -

Spin the Evidence of Intrinsic Angular Momentum Or Spin and Its
Spin The evidence of intrinsic angular momentum or spin and its associated magnetic moment came through experiments by Stern and Gerlach and works of Goudsmit and Uhlenbeck. The spin is called intrinsic since, unlike orbital angular momentum which is extrinsic, it is carried by point particle in addition to its orbital angular momentum and has nothing to do with motion in space. The magnetic moment ~µ of silver atom was measured in 1922 experiment by Stern and Gerlach and its projection µz in the direction of magnetic fieldz ^ B (through which the silver beam was passed) was found to be just −|~µj and +j~µj instead of continuously varying between these two as limits. Classically, magnetic moment is proportional to the angular momentum (12) and, assuming this proportionality to survive in quantum mechanics, quan- tization of magnetic moment leads to quantization of corresponding angular momentum S, which we called spin, q q µ = g S ) µ = g ~ m (57) z 2m z z 2m s as is the case with orbital angular momentum, where the ratio q=2m is called Bohr mag- neton, µb and g is known as Lande-g factor or gyromagnetic factor. The g-factor is 1 for orbital angular momentum and hence corresponding magnetic moment is l µb (where l is integer orbital angular momentum quantum number). A surprising feature here is that spin magnetic moment is also µb instead of µb=2 as one would naively expect. So it turns out g = 2 for spin { an effect that can only be understood if linearization of Schr¨odinger equation is attempted i.e. -

7. Examples of Magnetic Energy Diagrams. P.1. April 16, 2002 7
7. Examples of Magnetic Energy Diagrams. There are several very important cases of electron spin magnetic energy diagrams to examine in detail, because they appear repeatedly in many photochemical systems. The fundamental magnetic energy diagrams are those for a single electron spin at zero and high field and two correlated electron spins at zero and high field. The word correlated will be defined more precisely later, but for now we use it in the sense that the electron spins are correlated by electron exchange interactions and are thereby required to maintain a strict phase relationship. Under these circumstances, the terms singlet and triplet are meaningful in discussing magnetic resonance and chemical reactivity. From these fundamental cases the magnetic energy diagram for coupling of a single electron spin with a nuclear spin (we shall consider only couplings with nuclei with spin 1/2) at zero and high field and the coupling of two correlated electron spins with a nuclear spin are readily derived and extended to the more complicated (and more realistic) cases of couplings of electron spins to more than one nucleus or to magnetic moments generated from other sources (spin orbit coupling, spin lattice coupling, spin photon coupling, etc.). Magentic Energy Diagram for A Single Electron Spin and Two Coupled Electron Spins. Zero Field. Figure 14 displays the magnetic energy level diagram for the two fundamental cases of : (1) a single electron spin, a doublet or D state and (2) two correlated electron spins, which may be a triplet, T, or singlet, S state. In zero field (ignoring the electron exchange interaction and only considering the magnetic interactions) all of the magnetic energy levels are degenerate because there is no preferred orientation of the angular momentum and therefore no preferred orientation of the magnetic moment due to spin. -

High Pressure Band Structure, Density of States, Structural Phase Transition and Metallization in Cds
Chemical and Materials Engineering 5(1): 8-13, 2017 http://www.hrpub.org DOI: 10.13189/cme.2017.050102 High Pressure Band Structure, Density of States, Structural Phase Transition and Metallization in CdS J. Jesse Pius1, A. Lekshmi2, C. Nirmala Louis2,* 1Rohini College of Engineering, Nagercoil, Kanyakumari District, India 2Research Center in Physics, Holy Cross College, India Copyright©2017 by authors, all rights reserved. Authors agree that this article remains permanently open access under the terms of the Creative Commons Attribution License 4.0 International License Abstract The electronic band structure, density of high pressure studies due to the development of different states, metallization and structural phase transition of cubic designs of diamond anvil cell (DAC). With a modern DAC, zinc blende type cadmium sulphide (CdS) is investigated it is possible to reach pressures of 2 Mbar (200 GPa) using the full potential linear muffin-tin orbital (FP-LMTO) routinely and pressures of 5 Mbar (500 GPa) or higher is method. The ground state properties and band gap values achievable [3]. At such pressures, materials are reduced to are compared with the experimental results. The fractions of their original volumes. With this reduction in equilibrium lattice constant, bulk modulus and its pressure inter atomic distances; significant changes in bonding and derivative and the phase transition pressure at which the structure as well as other properties take place. The increase compounds undergo structural phase transition from ZnS to of pressure means the significant decrease in volume, which NaCl are predicted from the total energy calculations. The results in the change of electronic states and crystal structure. -

Study of Spin Glass and Cluster Ferromagnetism in Rusr2eu1.4Ce0.6Cu2o10-Δ Magneto Superconductor Anuj Kumar, R
Study of spin glass and cluster ferromagnetism in RuSr2Eu1.4Ce0.6Cu2O10-δ magneto superconductor Anuj Kumar, R. P. Tandon, and V. P. S. Awana Citation: J. Appl. Phys. 110, 043926 (2011); doi: 10.1063/1.3626824 View online: http://dx.doi.org/10.1063/1.3626824 View Table of Contents: http://jap.aip.org/resource/1/JAPIAU/v110/i4 Published by the American Institute of Physics. Related Articles Annealing effect on the excess conductivity of Cu0.5Tl0.25M0.25Ba2Ca2Cu3O10−δ (M=K, Na, Li, Tl) superconductors J. Appl. Phys. 111, 053914 (2012) Effect of columnar grain boundaries on flux pinning in MgB2 films J. Appl. Phys. 111, 053906 (2012) The scaling analysis on effective activation energy in HgBa2Ca2Cu3O8+δ J. Appl. Phys. 111, 07D709 (2012) Magnetism and superconductivity in the Heusler alloy Pd2YbPb J. Appl. Phys. 111, 07E111 (2012) Micromagnetic analysis of the magnetization dynamics driven by the Oersted field in permalloy nanorings J. Appl. Phys. 111, 07D103 (2012) Additional information on J. Appl. Phys. Journal Homepage: http://jap.aip.org/ Journal Information: http://jap.aip.org/about/about_the_journal Top downloads: http://jap.aip.org/features/most_downloaded Information for Authors: http://jap.aip.org/authors Downloaded 12 Mar 2012 to 14.139.60.97. Redistribution subject to AIP license or copyright; see http://jap.aip.org/about/rights_and_permissions JOURNAL OF APPLIED PHYSICS 110, 043926 (2011) Study of spin glass and cluster ferromagnetism in RuSr2Eu1.4Ce0.6Cu2O10-d magneto superconductor Anuj Kumar,1,2 R. P. Tandon,2 and V. P. S. Awana1,a) 1Quantum Phenomena and Application Division, National Physical Laboratory (CSIR), Dr. -
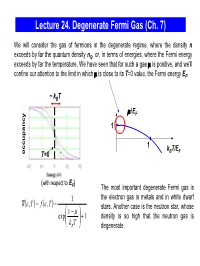
Lecture 24. Degenerate Fermi Gas (Ch
Lecture 24. Degenerate Fermi Gas (Ch. 7) We will consider the gas of fermions in the degenerate regime, where the density n exceeds by far the quantum density nQ, or, in terms of energies, where the Fermi energy exceeds by far the temperature. We have seen that for such a gas μ is positive, and we’ll confine our attention to the limit in which μ is close to its T=0 value, the Fermi energy EF. ~ kBT μ/EF 1 1 kBT/EF occupancy T=0 (with respect to E ) F The most important degenerate Fermi gas is 1 the electron gas in metals and in white dwarf nε()(),, T= f ε T = stars. Another case is the neutron star, whose ε⎛ − μ⎞ exp⎜ ⎟ +1 density is so high that the neutron gas is ⎝kB T⎠ degenerate. Degenerate Fermi Gas in Metals empty states ε We consider the mobile electrons in the conduction EF conduction band which can participate in the charge transport. The band energy is measured from the bottom of the conduction 0 band. When the metal atoms are brought together, valence their outer electrons break away and can move freely band through the solid. In good metals with the concentration ~ 1 electron/ion, the density of electrons in the electron states electron states conduction band n ~ 1 electron per (0.2 nm)3 ~ 1029 in an isolated in metal electrons/m3 . atom The electrons are prevented from escaping from the metal by the net Coulomb attraction to the positive ions; the energy required for an electron to escape (the work function) is typically a few eV. -

Lecture Notes for Quantum Matter
Lecture Notes for Quantum Matter MMathPhys c Professor Steven H. Simon Oxford University July 24, 2019 Contents 1 What we will study 1 1.1 Bose Superfluids (BECs, Superfluid He, Superconductors) . .1 1.2 Theory of Fermi Liquids . .2 1.3 BCS theory of superconductivity . .2 1.4 Special topics . .2 2 Introduction to Superfluids 3 2.1 Some History and Basics of Superfluid Phenomena . .3 2.2 Landau and the Two Fluid Model . .6 2.2.1 More History and a bit of Physics . .6 2.2.2 Landau's Two Fluid Model . .7 2.2.3 More Physical Effects and Their Two Fluid Pictures . .9 2.2.4 Second Sound . 12 2.2.5 Big Questions Remaining . 13 2.3 Curl Free Constraint: Introducing the Superfluid Order Parameter . 14 2.3.1 Vorticity Quantization . 15 2.4 Landau Criterion for Superflow . 17 2.5 Superfluid Density . 20 2.5.1 The Andronikoshvili Experiment . 20 2.5.2 Landau's Calculation of Superfluid Density . 22 3 Charged Superfluid ≈ Superconductor 25 3.1 London Theory . 25 3.1.1 Meissner-Ochsenfeld Effect . 27 3 3.1.2 Quantum Input and Superfluid Order Parameter . 29 3.1.3 Superconducting Vortices . 30 3.1.4 Type I and Type II superconductors . 32 3.1.5 How big is Hc ............................... 33 4 Microscopic Theory of Bosons 37 4.1 Mathematical Preliminaries . 37 4.1.1 Second quantization . 37 4.1.2 Coherent States . 38 4.1.3 Multiple orbitals . 40 4.2 BECs and the Gross-Pitaevskii Equation . 41 4.2.1 Noninteracting BECs as Coherent States .