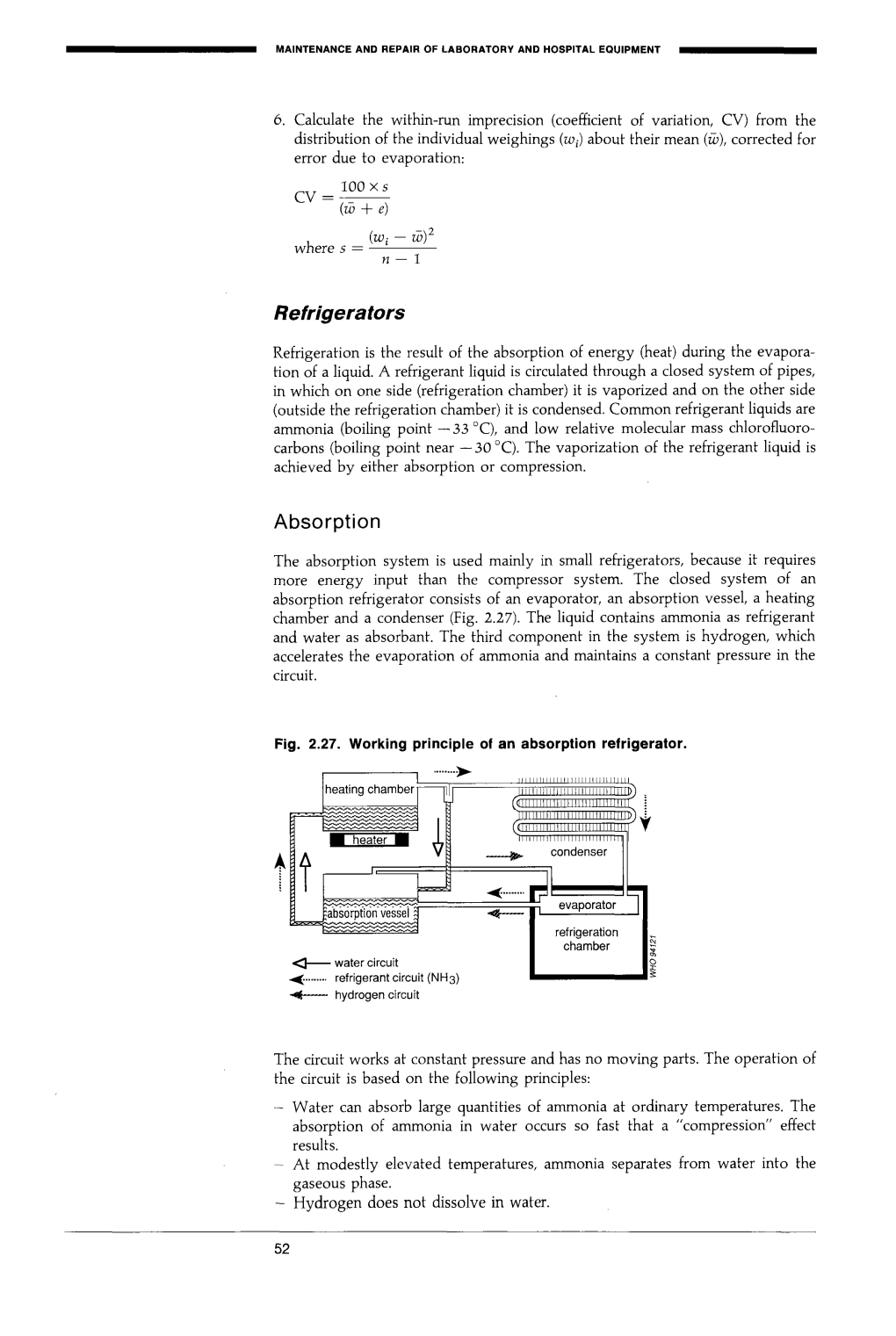
Refrigerators Absorption
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heat Transfer Model of a Rotary Compressor S
Purdue University Purdue e-Pubs International Compressor Engineering Conference School of Mechanical Engineering 1992 Heat Transfer Model of a Rotary Compressor S. K. Padhy General Electric Company Follow this and additional works at: https://docs.lib.purdue.edu/icec Padhy, S. K., "Heat Transfer Model of a Rotary Compressor" (1992). International Compressor Engineering Conference. Paper 935. https://docs.lib.purdue.edu/icec/935 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Complete proceedings may be acquired in print and on CD-ROM directly from the Ray W. Herrick Laboratories at https://engineering.purdue.edu/ Herrick/Events/orderlit.html HEAT TRANSFER MODEL OF A ROTARY COMPRESSOR Sisir K. Padhy General Electric C_ompany Appliance Park 5-2North, Louisville, KY 40225 ABSTRACT Energy improvements for a rotary compressor can be achieved in several ways such as: reduction of various electrical and mechanical losses, reduction of gas leakage, better lubrication, better surface cooling, reduction of suction gas heating and by improving other parameters. To have a ' better understanding analytical/numerical analysis is needed. Although various mechanical models are presented to understand the mechanical losses, dynamics, thermodynamics etc.; little work has been done to understand the compressor from a heat uansfer stand point In this paper a lumped heat transfer model for the rotary compressor is described. Various heat sources and heat sinks are analyzed and the temperature profile of the compressor is generated. A good agreement is found between theoretical and experimental results. NOMENCLATURE D, inner diameter D. -

Why Does the Wisconsin Boiler and Pressure Vessel Code, 636 41
Why does the Wisconsin Boiler and Pressure Vessel Code, SPS 341, regulate air compressors? By Industry Services Division Boiler and Pressure Vessels Program Almost everyone forms a definite picture in their mind when they hear the term “air compressor.” They probably think of a steel tank of some shape and dimension, with an electric motor, and an air pump mounted on top of the tank. To better understand the requirements of the Wisconsin Boiler Code, SPS 341, pertaining to air compressors, we need to have some common terminology. If a person wanted to purchase an “air compressor” at a hardware or home supply store, they would certainly be offered the equipment described above, consisting of three separate mechanical devices; the motor, the pump, and the storage tank. First, there is an electric motor, used to provide power to the device that pumps air to high pressure. This pumping device is the only part of the unit that is properly called an "air compressor." The compressor delivers high-pressure air to the third and final part of the mechanical assembly, the air storage tank; commonly called a pressure vessel. Because the pressure vessel receives pressurized air, this is the only part of the device described above that is regulated SPS 341. SPS 341 regulates pressure vessels when the pressure vessel's volume capacity is 90 gallons (12 cubic feet) or larger, and the air pressure is 15 pounds per square inch or higher. A typical pressure vessel of this size would be 24 inches in diameter and 48 inches long. In industrial settings, it is common practice to mount very large air compressors in a separate location from the pressure vessels. -

Performance Comparison Between an Absorption-Compression Hybrid Refrigeration System and a Double-Effect Absorption Refrigeration Sys-Tem
Available online at www.sciencedirect.com ScienceDirect Available online at www.sciencedirect.com Procedia Engineering 00 (2017) 000–000 ScienceDirect www.elsevier.com/locate/procedia Procedia Engineering 205 (2017) 241–247 10th International Symposium on Heating, Ventilation and Air Conditioning, ISHVAC2017, 19- 22 October 2017, Jinan, China Performance Comparison between an Absorption-compression Hybrid Refrigeration System and a Double-effect Absorption Refrigeration Sys-tem Jian Wang, Xianting Li, Baolong Wang, Wei Wu, Pengyuan Song, Wenxing Shi* Beijing Key Laboratory of Indoor Air Quality Evaluation and Control, Department of Building Science, Tsinghua University, Haidian District, Beijing 100084, China Abstract Conventional absorption refrigeration systems (ARSs), which are mainly driven by heat, have a competitive primary energy efficiency (PEE) compared with chillers driven by electricity. In the typical ARS, a large quantity of high-temperature heat is supplied into the generator, while a substantial amount of low-temperature heat is rejected to the environment from the condenser, it’s a huge waste. In order to decrease the input heat for generator and enhance the performance of conventional ARS, a kind of absorption-compression hybrid refrigeration system recovering condensation heat for generation (RCHG-ARS) was ever proposed. In the present study, the models of both RCHG-ARS and double-effect absorption refrigeration system (DEARS) are established, and the effects of different parameters on them are simulated and compared with each other. As a conclusion, the PEE of RCHG-ARS can be 29.0% higher than that of DEARS, and RCHG-ARS has a wider working conditions than DEARS due to the existence of the compressor. -

Bendix® Ba-922® Sae Universal Flange Air Compressor
compressors BENDIX® BA-922® SAE UNIVERSAL FLANGE AIR COMPRESSOR High output compressed air generation for ultra demanding commercial & industrial applications. Experience Counts Ideal for niche industrial, agricultural, and For generations, Bendix Commercial Vehicle Systems (Bendix CVS) has petroleum applications, the universal flange been leading the way in air charging system experience and expertise. model . More fleets specify our hard-working products and systems than any other. Features an adapter mount to meet SAE J744 Bendix CVS continues its legacy of quality, reliability and durability with hydraulic pump, engine & motor mounting, a next generation high-performing, energy-saving compressor option – and drive dimensions standards; the Bendix® BA-922® SAE Universal Flange compressor. Can be mounted at several different angles An Ideal Solution For A Wide Range Of depending on physical constraints and application necessities; and Unique Applications Utilizing a standard SAE B Flange, and a 15-tooth BB spline for strength, Is driven by an engine PTO, hydraulic motor, the Bendix® BA-922® SAE compressor is designed to address a wide or electric motor. Option for belt drive as well. variety of commercial and industrial applications. Its clock-able front adapter allows the compressor to be easily mounted in several different positions depending on your requirement. Building On The Power Of The Bendix® BA-922® Compressor: High Air Delivery A dependable workhorse, the Bendix® BA-922® compressor provides maximum air delivery even at low speeds. Its high-output, two-cylinder design supplies 32 cfm (cubic feet per minute) displacement at 1,250 rpm (revolutions per minute). Power like this delivers the ability to recharge the system quickly and efficiently – even at low speeds – making it ideal for more demanding brake system applications. -

Solar Heating and Cooling & Solar Air-Conditioning Position Paper
Task 53 New Generation Solar Cooling & Heating Systems (PV or solar thermally driven systems) Solar Heating and Cooling & Solar Air-Conditioning Position Paper November 2018 Contents Executive Summary ............................................................. 3 Introduction and Relevance ................................................ 4 Status of the Technology/Industry ...................................... 5 Technical maturity and basic successful rules for design .............. 7 Energy performance for PV and Solar thermally driven systems ... 8 Economic viability and environmental benefits .............................. 9 Market status .................................................................................... 9 Potential ............................................................................. 10 Technical potential ......................................................................... 10 Costs and economics ..................................................................... 11 Market opportunities ...................................................................... 12 Current Barriers ................................................................. 12 Actions Needed .................................................................. 13 This document was prepared by Daniel Neyer1,2 and Daniel Mugnier3 with support by Alexander Thür2, Roberto Fedrizzi4 and Pedro G. Vicente Quiles5. 1 daniel neyer brainworks, Oberradin 50, 6700 Bludenz, Austria 2 University of Innsbruck, Technikerstr. 13, 6020 Innsbruck, Austria -

Chapter 8 and 9 – Energy Balances
CBE2124, Levicky Chapter 8 and 9 – Energy Balances Reference States . Recall that enthalpy and internal energy are always defined relative to a reference state (Chapter 7). When solving energy balance problems, it is therefore necessary to define a reference state for each chemical species in the energy balance (the reference state may be predefined if a tabulated set of data is used such as the steam tables). Example . Suppose water vapor at 300 oC and 5 bar is chosen as a reference state at which Hˆ is defined to be zero. Relative to this state, what is the specific enthalpy of liquid water at 75 oC and 1 bar? What is the specific internal energy of liquid water at 75 oC and 1 bar? (Use Table B. 7). Calculating changes in enthalpy and internal energy. Hˆ and Uˆ are state functions , meaning that their values only depend on the state of the system, and not on the path taken to arrive at that state. IMPORTANT : Given a state A (as characterized by a set of variables such as pressure, temperature, composition) and a state B, the change in enthalpy of the system as it passes from A to B can be calculated along any path that leads from A to B, whether or not the path is the one actually followed. Example . 18 g of liquid water freezes to 18 g of ice while the temperature is held constant at 0 oC and the pressure is held constant at 1 atm. The enthalpy change for the process is measured to be ∆ Hˆ = - 6.01 kJ. -

Ammonia As a Refrigerant
1791 Tullie Circle, NE. Atlanta, Georgia 30329-2305, USA www.ashrae.org ASHRAE Position Document on Ammonia as a Refrigerant Approved by ASHRAE Board of Directors February 1, 2017 Expires February 1, 2020 ASHRAE S H A P I N G T O M O R R O W ’ S B U I L T E N V I R O N M E N T T O D A Y © 2017 ASHRAE (www.ashrae.org). For personal use only. Additional reproduction, distribution, or transmission in either print or digital form is not permitted without ASHRAE’s prior written permission. COMMITTEE ROSTER The ASHRAE Position Document on “Ammonia as a Refrigerant” was developed by the Society’s Refrigeration Committee. Position Document Committee formed on January 8, 2016 with Dave Rule as its chair. Dave Rule, Chair Georgi Kazachki IIAR Dayton Phoenix Group Alexandria, VA, USA Dayton, OH, USA Ray Cole Richard Royal Axiom Engineers, Inc. Walmart Monterey, CA, USA Bentonville, Arkansas, USA Dan Dettmers Greg Scrivener IRC, University of Wisconsin Cold Dynamics Madison, WI, USA Meadow Lake, SK, Canada Derek Hamilton Azane Inc. San Francisco, CA, USA Other contributors: M. Kent Anderson Caleb Nelson Consultant Azane, Inc. Bethesda, MD, USA Missoula, MT, USA Cognizant Committees The chairperson of Refrigerant Committee also served as ex-officio members: Karim Amrane REF Committee AHRI Bethesda, MD, USA i © 2017 ASHRAE (www.ashrae.org). For personal use only. Additional reproduction, distribution, or transmission in either print or digital form is not permitted without ASHRAE’s prior written permission. HISTORY of REVISION / REAFFIRMATION / WITHDRAWAL -

Power Generation Technology by Hot Water Heating of Low Temperature Power Generations Using Ammonia and Ammonia-Water Mixture As Working Fluid
Proceedings of the 6th Asian Geothermal Symposium, Oct. 26-29, 2004 Mutual Challenges in High- and Low- Temperature Geothermal Resource Fields, 5-7 POWER GENERATION TECHNOLOGY BY HOT WATER HEATING OF LOW TEMPERATURE POWER GENERATIONS USING AMMONIA AND AMMONIA-WATER MIXTURE AS WORKING FLUID Takumi HASHIZUME Waseda Univ., 17 Kikui-cho, Shinjuku-ku, Tokyo, 162-0044, Japan e-mail:[email protected] 1. INTRODUCTION The heat discharged without being used for surroundings including the factory waste heat is not a little. It is widely used when the temperature of the heat source is high, and it is used comparatively effectively. On the other hand, the heat is not effectively used for the heat source of a low temperature of 100℃ or less. As for the geothermal energy, this is similar. It is used as a geothermal power generation when the steam of the high temperature and high pressure is obtained, and the usage is not wide for the steam and the hot water of the low temperature and low pressure. If the use as heat in the hot spring, the indoor air-conditioning (heating), the greenhouse, and the road heating, etc. is excluded for the hot water of 100℃ or less, it is hardly used in Japan. In this paper, it introduces the power generation technology which obtains electricity from the hot water of 100℃ or less by using the power generation cycle when the medium with a low boiling point (low boiling point medium) is made an working fluid. 2. HEAT RECOVERY AND RANKINE CYCLE BY LOW BOILING POINT MEDIUM Rankine cycle consists of the pump, the evaporator, the turbine, and the condenser, and the working fluid circulates around each equipment in this order. -

A Comprehensive Review of Thermal Energy Storage
sustainability Review A Comprehensive Review of Thermal Energy Storage Ioan Sarbu * ID and Calin Sebarchievici Department of Building Services Engineering, Polytechnic University of Timisoara, Piata Victoriei, No. 2A, 300006 Timisoara, Romania; [email protected] * Correspondence: [email protected]; Tel.: +40-256-403-991; Fax: +40-256-403-987 Received: 7 December 2017; Accepted: 10 January 2018; Published: 14 January 2018 Abstract: Thermal energy storage (TES) is a technology that stocks thermal energy by heating or cooling a storage medium so that the stored energy can be used at a later time for heating and cooling applications and power generation. TES systems are used particularly in buildings and in industrial processes. This paper is focused on TES technologies that provide a way of valorizing solar heat and reducing the energy demand of buildings. The principles of several energy storage methods and calculation of storage capacities are described. Sensible heat storage technologies, including water tank, underground, and packed-bed storage methods, are briefly reviewed. Additionally, latent-heat storage systems associated with phase-change materials for use in solar heating/cooling of buildings, solar water heating, heat-pump systems, and concentrating solar power plants as well as thermo-chemical storage are discussed. Finally, cool thermal energy storage is also briefly reviewed and outstanding information on the performance and costs of TES systems are included. Keywords: storage system; phase-change materials; chemical storage; cold storage; performance 1. Introduction Recent projections predict that the primary energy consumption will rise by 48% in 2040 [1]. On the other hand, the depletion of fossil resources in addition to their negative impact on the environment has accelerated the shift toward sustainable energy sources. -

Investigating Absorption Refrigerator Fires (Part I)
Orion P. Keifer Peter D. Layson Charles A. Wensley Investigating Absorption Refrigerator Fires (Part I) ATLANTIC BEACH, FLORIDA—In today’s recreational vehicles (RV), the then expels it when perco- most common refrigerator uses absorption refrigeration technology, lated in the boiler. It is this primarily because this type of system can operate on multiple sources action of the water which of power, including propane when electrical power is unavailable. makes the ammonia flow. These refrigerators have been under intense scrutiny in recent years The hydrogen in the re- due to numerous reported fires, apparently starting in the area of the frigeration coil maintains absorption refrigerator. Both the Dometic Corporation and Norcold a positive pressure of ap- Incorporated, two manufacturers of RV refrigerators, have been re- proximately 300-375 PSI quired by the National Highway and Traffic Safety Administration (2.07-2.59 MPa) when (NHTSA) to recall certain models of refrigerators which have been not in operation and, due identified as capable of failing in a fire mode. In summary, the three to its low partial pressure, NHTSA recalls indicate a fatigue crack may develop in the boiler tube promotes the evaporation of the cooling unit which may release sufficient pressurized flammable of the liquid ammonia. It coolant solution into an area where an ignition source is present. The should be noted that unlike NHTSA Recall Campaign ID Numbers are 06E076000 for Dometic conventional refrigeration (926,877 affected units), and 02E019000 (28,144 affected units) and systems which extensively 02E045000 (8,419 affected units) for Norcold. use copper due to its high thermal conductivity, the Applications Engineering Group, Inc. -

Solar Air-Conditioning and Refrigeration - Achievements and Challenges
Solar air-conditioning and refrigeration - achievements and challenges Hans-Martin Henning Fraunhofer-Institut für Solare Energiesysteme ISE, Freiburg/Germany EuroSun 2010 September 28 – October 2, 2010 Graz - AUSTRIA © Fraunhofer ISE Outline Components and systems Achievements Solar thermal versus PV? Challenges and conclusion © Fraunhofer ISE Components and systems Achievements Solar thermal versus PV? Challenges and conclusion © Fraunhofer ISE Overall approach to energy efficient buildings Assure indoor comfort with a minimum energy demand 1. Reduction of energy demand Building envelope; ventilation 2. Use of heat sinks (sources) in Ground; outside air (T, x) the environment directly or indirectly; storage mass 3. Efficient conversion chains HVAC; combined heat, (minimize exergy losses) (cooling) & power (CH(C)P); networks; auxiliary energy 4. (Fractional) covering of the Solar thermal; PV; (biomass) remaining demand using renewable energies © Fraunhofer ISE Solar thermal cooling - basic principle Basic systems categories Closed cycles (chillers): chilled water Open sorption cycles: direct treatment of fresh air (temperature, humidity) © Fraunhofer ISE Open cycles – desiccant air handling units Solid sorption Liquid sorption Desiccant wheels Packed bed Coated heat exchangers Plate heat exchanger Silica gel or LiCl-matrix, future zeolite LiCl-solution: Thermochemical storage possible ECOS (Fraunhofer ISE) in TASK 38 © Fraunhofer ISE Closed cycles – water chillers or ice production Liquid sorption: Ammonia-water or Water-LiBr (single-effect or double-effect) Solid sorption: silica gel – water, zeolite-water Ejector systems Thermo-mechanical systems Turbo Expander/Compressor AC-Sun, Denmark in TASK 38 © Fraunhofer ISE System overview Driving Collector type System type temperature Low Open cycle: direct air treatment (60-90°C) Closed cycle: high temperature cooling system (e.g. -

Empirical Modelling of Einstein Absorption Refrigeration System
Journal of Advanced Research in Fluid Mechanics and Thermal Sciences 75, Issue 3 (2020) 54-62 Journal of Advanced Research in Fluid Mechanics and Thermal Sciences Journal homepage: www.akademiabaru.com/arfmts.html ISSN: 2289-7879 Empirical Modelling of Einstein Absorption Refrigeration Open Access System Keng Wai Chan1,*, Yi Leang Lim1 1 School of Mechanical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia ARTICLE INFO ABSTRACT Article history: A single pressure absorption refrigeration system was invented by Albert Einstein and Received 4 April 2020 Leo Szilard nearly ninety-year-old. The system is attractive as it has no mechanical Received in revised form 27 July 2020 moving parts and can be driven by heat alone. However, the related literature and Accepted 5 August 2020 work done on this refrigeration system is scarce. Previous researchers analysed the Available online 20 September 2020 refrigeration system theoretically, both the system pressure and component temperatures were fixed merely by assumption of ideal condition. These values somehow have never been verified by experimental result. In this paper, empirical models were proposed and developed to estimate the system pressure, the generator temperature and the partial pressure of butane in the evaporator. These values are important to predict the system operation and the evaporator temperature. The empirical models were verified by experimental results of five experimental settings where the power input to generator and bubble pump were varied. The error for the estimation of the system pressure, generator temperature and partial pressure of butane in evaporator are ranged 0.89-6.76%, 0.23-2.68% and 0.28-2.30%, respectively.