Nasal Turbinates and the Evolution of Mammalian Endothermy
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Therapsida: Dinocephalia) Christian F
This article was downloaded by: [University of Guelph] On: 30 April 2012, At: 12:37 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Journal of Systematic Palaeontology Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/tjsp20 Systematics of the Anteosauria (Therapsida: Dinocephalia) Christian F. Kammerer a a Department of Vertebrate Paleontology, American Museum of Natural History, New York, 10024-5192, USA Available online: 13 Dec 2010 To cite this article: Christian F. Kammerer (2011): Systematics of the Anteosauria (Therapsida: Dinocephalia), Journal of Systematic Palaeontology, 9:2, 261-304 To link to this article: http://dx.doi.org/10.1080/14772019.2010.492645 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. Journal of Systematic Palaeontology, Vol. -

Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships
438 Article 438 by Saswati Bandyopadhyay1* and Sanghamitra Ray2 Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships 1Geological Studies Unit, Indian Statistical Institute, 203 B. T. Road, Kolkata 700108, India; email: [email protected] 2Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur 721302, India; email: [email protected] *Corresponding author (Received : 23/12/2018; Revised accepted : 11/09/2019) https://doi.org/10.18814/epiiugs/2020/020028 The twelve Gondwanan stratigraphic horizons of many extant lineages, producing highly diverse terrestrial vertebrates India have yielded varied vertebrate fossils. The oldest in the vacant niches created throughout the world due to the end- Permian extinction event. Diapsids diversified rapidly by the Middle fossil record is the Endothiodon-dominated multitaxic Triassic in to many communities of continental tetrapods, whereas Kundaram fauna, which correlates the Kundaram the non-mammalian synapsids became a minor components for the Formation with several other coeval Late Permian remainder of the Mesozoic Era. The Gondwana basins of peninsular horizons of South Africa, Zambia, Tanzania, India (Fig. 1A) aptly exemplify the diverse vertebrate faunas found Mozambique, Malawi, Madagascar and Brazil. The from the Late Palaeozoic and Mesozoic. During the last few decades much emphasis was given on explorations and excavations of Permian-Triassic transition in India is marked by vertebrate fossils in these basins which have yielded many new fossil distinct taxonomic shift and faunal characteristics and vertebrates, significant both in numbers and diversity of genera, and represented by small-sized holdover fauna of the providing information on their taphonomy, taxonomy, phylogeny, Early Triassic Panchet and Kamthi fauna. -

Femur of a Morganucodontid Mammal from the Middle Jurassic of Central Russia
Femur of a morganucodontid mammal from the Middle Jurassic of Central Russia PETR P. GAMBARYAN and ALEXANDER 0.AVERIANOV Gambaryan, P.P. & Averianov, A.O. 2001. Femur of a morganucodontid mammal from the Middle Jurassic of Central Russia. -Acta Palaeontologica Polonica 46,1,99-112. We describe a nearly complete mammalian femur from the Middle Jurassic (upper Bathonian) from Peski quarry, situated some 100 km south east of Moscow, central Rus- sia. It is similar to the femora of Morganucodontidae in having a globular femoral head, separated from the greater trochanter and reflected dorsally, fovea capitis present, both trochanters triangular and located on the same plane, distal end flat, mediolaterally expanded, and somewhat bent ventrally, and in the shape and proportions of distal condyles. It is referred to as Morganucodontidae gen. et sp. indet. It is the first representa- tive of this group of mammals in Eastern Europe from the third Mesozoic mammal local- ity discovered in Russia. Exquisite preservation of the bone surface allowed us to recon- struct partial hind limb musculature. We reconstruct m. iliopsoas as inserting on the ridge, which starts at the lesser trochanter and extends along the medial femoral margin for more than half of the femur length. On this basis we conclude that the mode of loco- motion of the Peski morganucodontid was similar to that of modern echidnas. During the propulsive phase the femur did not retract and the step elongation was provided by pronation of the femur. Key words : Mammalia, Morganucodontidae, femur, anatomy, locomotion, Jurassic, Russia. Petr P. Gambaryan [[email protected]] and Alexander 0. -

A Non-Mammaliaform Cynodont from the Upper Triassic of South Africa: a Therapsid Lazarus Taxon?
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE A non-mammaliaform cynodont from the Upper Triassic of South Africa: a therapsid Lazarus taxon? Fernando Abdala1*, Ross Damiani2, Adam Yates1 & Johann Neveling3 1Bernard Price Institute for Palaeontological Research, School of Geosciences, University of the Witwatersrand, Private Bag 3, WITS, 2050 South Africa 2Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, D-70191, Stuttgart, Germany 3Council for Geoscience, Private Bag X112, Pretoria, 0001 South Africa Received 20 January 2006. Accepted 10 January 2007 The tetrapod record of the ‘Stormberg Group’, including the Lower Elliot Formation, in the South African Karoo is widely dominated by archosaurian reptiles, contrasting with the therapsid dominion of the subjacent Beaufort Group. The only therapsids represented by skeletal remains in the Upper Triassic Lower Elliot Formation are the large traversodontid cynodont Scalenodontoides macrodontes and the recently described tritheledontid cynodont Elliotherium kersteni. Here we present a fragmentary lower jaw that provides evidence of a third type of cynodont for the Upper Triassic of South Africa. The fossil is tentatively assigned to the Diademodontidae. The latter representative of this family is known from the Late Anisian, and its tentative record in the Norian Lower Elliot Formation, if confirmed, will represent a case of Lazarus taxon. Thus, Diademodontidae apparently disappeared from the fossil record by the end of the Anisian and then reappeared in the Norian of South Africa, a stratigraphic interval of some 21 million years. This new cynodont record, together with the recently described Tritheledontidae, show that cynodonts are now the second most diverse tetrapod group in the Lower Elliot fauna. -

Osteohistology of Late Triassic Prozostrodontian Cynodonts from Brazil
Osteohistology of Late Triassic prozostrodontian cynodonts from Brazil Jennifer Botha-Brink1,2, Marina Bento Soares3 and Agustín G. Martinelli3 1 Department of Karoo Palaeontology, National Museum, Bloemfontein, South Africa 2 Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa 3 Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil ABSTRACT The Prozostrodontia includes a group of Late Triassic-Early Cretaceous eucynodonts plus the clade Mammaliaformes, in which Mammalia is nested. Analysing their growth patterns is thus important for understanding the evolution of mammalian life histories. Obtaining material for osteohistological analysis is difficult due to the rare and delicate nature of most of the prozostrodontian taxa, much of which comprises mostly of crania or sometimes even only teeth. Here we present a rare opportunity to observe the osteohistology of several postcranial elements of the basal prozostrodontid Prozostrodon brasiliensis, the tritheledontid Irajatherium hernandezi, and the brasilodontids Brasilodon quadrangularis and Brasilitherium riograndensis from the Late Triassic of Brazil (Santa Maria Supersequence). Prozostrodon and Irajatherium reveal similar growth patterns of rapid early growth with annual interruptions later in ontogeny. These interruptions are associated with wide zones of slow growing bone tissue. Brasilodon and Brasilitherium exhibit a mixture of woven-fibered bone tissue and slower growing parallel-fibered and lamellar bone. The slower growing bone tissues are present even during early ontogeny. The relatively slower growth in Brasilodon and Brasilitherium may be related to their small body size compared to Prozostrodon and Irajatherium. These brasilodontids also exhibit osteohistological similarities with the Late Triassic/Early Jurassic mammaliaform Morganucodon and the Late Cretaceous multituberculate mammals Kryptobaatar and Nemegtbaatar. -

Early Cretaceous Amphilestid ('Triconodont') Mammals from Mongolia
Early Cretaceous amphilestid ('triconodont') mammals from Mongolia ZOFIAKIELAN-JAWOROWSKA and DEMBERLYIN DASHZEVEG Kielan-Jaworowską Z. &Daslueveg, D. 1998. Early Cretaceous amphilestid (.tricono- dont') mammals from Mongotia. - Acta Pal.aeontol.ogicaPolonica,43,3, 413438. Asmall collection of ?Aptianor ?Albian amphilestid('triconodont') mammals consisting of incomplete dentaries and maxillae with teeth, from the Khoboor localiĘ Guchin Us counĘ in Mongolia, is described. Grchinodon Troftmov' 1978 is regarded a junior subjective synonym of GobiconodonTroftmov, 1978. Heavier wear of the molariforms M3 andM4than of themore anteriorone-M2 in Gobiconodonborissiaki gives indirect evidence formolariformreplacement in this taxon. The interlocking mechanismbetween lower molariforms n Gobiconodon is of the pattern seen in Kuchneotherium and Ttnodon. The ińterlocking mechanism and the type of occlusion ally Amphilestidae with Kuehneotheriidae, from which they differ in having lower molariforms with main cusps aligned and the dentary-squamosal jaw joint (double jaw joint in Kuehneotheńdae). The main cusps in upper molariforms M3-M5 of Gobiconodon, however, show incipient tńangular arrangement. The paper gives some support to Mills' idea on the therian affinities of the Amphilestidae, although it cannot be excluded that the characters that unite the two groups developed in parallel. Because of scanty material and arnbiguĘ we assign the Amphilestidae to order incertae sedis. Key words : Mammali4 .triconodonts', Amphilestidae, Kuehneotheriidae, Early Cretaceous, Mongolia. Zofia Kiel,an-Jaworowska [zkielnn@twarda,pan.pl], InsĘtut Paleobiologii PAN, ul. Twarda 5 I /5 5, PL-00-8 I 8 Warszawa, Poland. DemberĘin Dash7eveg, Geological Institute, Mongolian Academy of Sciences, Ulan Bator, Mongolia. Introduction Beliajeva et al. (1974) reportedthe discovery of Early Cretaceous mammals at the Khoboor locality (referred to also sometimes as Khovboor), in the Guchin Us Soinon (County), Gobi Desert, Mongolia. -

Physical and Environmental Drivers of Paleozoic Tetrapod Dispersal Across Pangaea
ARTICLE https://doi.org/10.1038/s41467-018-07623-x OPEN Physical and environmental drivers of Paleozoic tetrapod dispersal across Pangaea Neil Brocklehurst1,2, Emma M. Dunne3, Daniel D. Cashmore3 &Jӧrg Frӧbisch2,4 The Carboniferous and Permian were crucial intervals in the establishment of terrestrial ecosystems, which occurred alongside substantial environmental and climate changes throughout the globe, as well as the final assembly of the supercontinent of Pangaea. The fl 1234567890():,; in uence of these changes on tetrapod biogeography is highly contentious, with some authors suggesting a cosmopolitan fauna resulting from a lack of barriers, and some iden- tifying provincialism. Here we carry out a detailed historical biogeographic analysis of late Paleozoic tetrapods to study the patterns of dispersal and vicariance. A likelihood-based approach to infer ancestral areas is combined with stochastic mapping to assess rates of vicariance and dispersal. Both the late Carboniferous and the end-Guadalupian are char- acterised by a decrease in dispersal and a vicariance peak in amniotes and amphibians. The first of these shifts is attributed to orogenic activity, the second to increasing climate heterogeneity. 1 Department of Earth Sciences, University of Oxford, South Parks Road, Oxford OX1 3AN, UK. 2 Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstraße 43, 10115 Berlin, Germany. 3 School of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham B15 2TT, UK. 4 Institut -

Mesozoic: the Dark Age for Mammals!
Ed’s Simplified History of the Mammals Note progression from Pelycosaurs (1) to Therapsids and Cynodonts (2) in Triassic. Stem mammals appeared in Late Triassic and Early Jurassic (3). Relationships among the Middle Jurassic forms (4) are controversial (see handout). Therian clade, identified by the tribosphenic molar (5), emerged at the end of the Jurassic, Early Cretaceous. A slightly more detailed version… in case you like something that looks more slick From Pough et al. 2009. Vertebrate Life, 8th ed. Pelycosaurs Dominated the late Permian, gave rise to therapsids Therapsids Rapid radiation in late Permian, around 270 MYA Still “mammal-like reptiles” The mass extinction at the end of the Permian was the greatest loss of diversity ever with >80% of all known genera and about 90% of all species going extinct, both terrestrial and marine. Cynodonts Late Permian to mid Triassic Last remaining group of therapsids, survived mass extinction at the end of the Permian. Persisted well Only 1 lineage of into Triassic and developed cynodonts survived many features associated through the late Triassic, with mammals. and this group became ancestors of mammals. Mesozoic: the Dark Age for Mammals! multituberculate Morganucodon, one of the earliest mammals (What else was happening in the Late Triassic and Jurassic Hadrocodium that may have contributed to mammals becoming small and Most were very small with nocturnal?) conservative morphology ...but new fossil finds indicate more diversity than we thought Repenomanus Still, largest known mammal during Mesozic Most were shrew to is no larger than a mouse sized, for 125 woodchuck million years! Some Mesozoic events and mammals you should know 1. -

Brief Report Vol
Brief report Vol. 46, No. 3, pp. 427-430, Warszawa 2001 Docodont nature of Cyrtlatherium, an upper Bathonian mammal from England DENISE SIGOGNEAU-RUSSELL The upper Bathonian Kirtlington mammal bed of England is one of the rare Middle Jurassic sites in the world to have yielded mammalian remains and undoubtedly the richest. A few taxa have so far been described, among which the genus Cyrtlatherium Freeman, 1979, assigned by its author to the Kuehneotheriidae and subsequently included in the Symmetrodonta. In this note I argue that Cyrtlathen'um represents a docodont of which it is possibly a milk premolar. In 1979, Freeman created the genus Cyrtlatherium on one complete right lower cheek tooth and two fragmentary ones from the Kirtlington mammal bed in Oxfordshire. His brief description was en- tirely done with reference to the lower molar of Kuehneotherium, without further justification of its systematic placement. His accompanying comments concern only the situation of Kuehneotherium among the Theria. The genus has since been regarded as a member of Symmetrodonta and was classi- fied as such in McKenna & Bell (1997). Reexamination of the holotype led to the conclusion that the tooth pertains in fact to the Docodonta, of which it presents all the main characteristics. Given the contradictions in the literature concerning the homologies of docodont molar cusps (see Butler 1997 and Sigogneau-Russell & Godefroit 1997 for the most recent proposals), and pending a further study, I follow Kermack et al. (1987: p. 4) in naming 'the cusps ... strictly according to their position in the teeth'. In accordance with this method, however, and differently from Kermack et al. -

Does the Jurassic Agilodocodon (Mammaliaformes, Docodonta) Have Any Exudativorous Dental Features?
DOES THE JURASSIC AGILODOCODON (MAMMALIAFORMES, DOCODONTA) HAVE ANY EXUDATIVOROUS DENTAL FEATURES? JOHN R. WIBLE and ANNE M. BURROWS Wible, J.R. and Burrows, A.M. 2016. Does the Jurassic Agilodocodon (Mammaliaformes, Docodonta) have any exudativorous dental features? Palaeontologia Polonica 67, 289–299. Obligate exudativory, including active wounding of bark to acquire gum and/or sap, is rare among extant mammals and does not show a consistent dental signature. A recently de- scribed Middle Jurassic docodont Agilodocodon was reconstructed as an exudativore based on proposed similarities of its lower anterior dentition to some extant New World monkeys, specifically marmosets, spider monkeys, and howler monkeys. Oddly enough, of these, only marmosets are exudate-feeders. In our reinvestigation, we did not find any significant resemblance in the lower (and upper) anterior dentition between the Middle Jurassic fossil and these extant New World monkeys. The marmosets, the only obligate platyrrhine exuda- tivores, have lower and upper incisors that are distinguished from Agilodocodon and other New World monkeys by having enamel restricted to the labial surface. Differential wear between the enamel and softer dentine maintains a chisel-like tooth that marmosets use in gouging bark. Additional comparisons of the anterior dentition of Agilodocodon and other extant mammals were conducted. The lower and upper anterior teeth of Agilodocodon were found to be most similar to some elephant shrews and South American marsupials, which have a primarily insectivorous diet. Agilodocodon does not show any dental adaptations found in extant mammals for exudativory. Key words: Docodonta, Agilodocodon, marmosets, exudativory, incisors, gums. John R. Wible [[email protected]], Section of Mammals, Carnegie Museum of Natural History, 5800 Baum Boulevard, Pittsburgh, PA 15206, USA. -

Geological Range Distinctive Characteristics and Phylogeny
Docodonta: The Non-Mammal Mammals GEOL 204 The Fossil Record Spring 2020 Section 0101 Anna Rose Barrack, Emily Feigenbaum, Phat Vu, Kayla Devaney Geological Range Ancestral Relations The Docodonta were a group of mammals that lived during the Mesozoic Era as seen in Figure D. Docodonts Figure E. The phylogenetic tree demonstrates that the clade of Docodonta is most closely related to Tikitherium, Woutersia, were first found in the Mid Jurrassic in the Bathonian of the UK and Russia. During the Late Jurassic many and Delsatia. (Panciroli et al., 16) fossils were found across Laurasia. It would further increase into the Cretaceous when another taxon was found in Russia. (Pancoroli et al., 2) Distinctive Characteristics and Phylogeny Speciation Figure G. The Castorocauda is a species of Docodonta that was semi-aquatic and burrowed. The holotype is the first fossil Figure B: Docodonts were distinctive from other mammalian forms due to their unique molar structure. This found of this species and is used to identify other fossils as this specimen. This species was very similar to that of the figure depicts multiple diagrams of upper and lower molars of the Docodon. These molars were unique from modern-day beaver. other mammaliaform, in that they were capable of both shearing and grinding their food. Figure H. The Agilodocodon is a species of Docodonta that is a little more unique. Rather than being semi-aquatic, it is Phylogenetic relationships have been able to be made to Docodon solely based on the structure of the molars. - thought to have been a tree climber. This has been hypothesized by comparing the phalanges, specifically the humerus bone Docodonta also had very unique living conditions as well, fossils indicate they were aboreal as well as semi located in the forearm, of the Agilodocodon with it’s swimming relative the Castorocauda. -
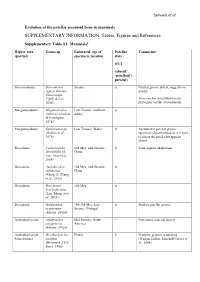
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp.