Reproductive Character Displacement in Calopteryx Aequabilis and C
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Olive Clubtail (Stylurus Olivaceus) in Canada, Prepared Under Contract with Environment Canada
COSEWIC Assessment and Status Report on the Olive Clubtail Stylurus olivaceus in Canada ENDANGERED 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olive Clubtail Stylurus olivaceus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 58 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge Robert A. Cannings, Sydney G. Cannings, Leah R. Ramsay and Richard J. Cannings for writing the status report on Olive Clubtail (Stylurus olivaceus) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Paul Catling, Co-chair of the COSEWIC Arthropods Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le gomphe olive (Stylurus olivaceus) au Canada. Cover illustration/photo: Olive Clubtail — Photo by Jim Johnson. Permission granted for reproduction. ©Her Majesty the Queen in Right of Canada, 2011. Catalogue No. CW69-14/637-2011E-PDF ISBN 978-1-100-18707-5 Recycled paper COSEWIC Assessment Summary Assessment Summary – May 2011 Common name Olive Clubtail Scientific name Stylurus olivaceus Status Endangered Reason for designation This highly rare, stream-dwelling dragonfly with striking blue eyes is known from only 5 locations within three separate regions of British Columbia. -

Catalogue of the Odonata of Michigan
MISCELLANEOUS PUBLICATIOKS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 104 Catalogue of the Odonata of Michigan EDWARD J. KORMOlVDY Department of Zoology, Oberlin College, Ohio ANN ARBOR MUSEUM OF ZOOLOGY, UNIVERSlTY OF MICHIGAN DECEMBER 17, 1958 LIST OF THE MISCELLANEOUS PUBLICATIONS OF THE MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN Address inquiries to the Director of the Museum of Zoology, Ann Arbor, Michigan Bound in Paper No. 1. Directions for Collecting and Preserving Specimens of Dragonflies for Museum Purposes. By E. B. Williamson. (1916) Pp. 15, 3 figures. .................... No. 2. An Annotated List of the Odonata of Indiana. By E. B. Williamson. (1917) Pp. 12, lmap ........................................................ No. 3. A Collecting Trip to Colombia, South America. By E. B. Williamson. (1918) Pp. 24 (Out of print) No. 4. Contributions to the Botany of Michigan. By C. K. Dodge. (1918) Pp. 14 ............. No. 5. Contributions to the Botany of Michigan, II. By C. K. Dodge. (1918) Pp. 44, 1 map. ..... No. 6. A Synopsis of the Classification of the Fresh-water Mollusca of North America, North of Mexico, and a Catalogue of the More Recently Described Species, with Notes. By Bryant Walker. (1918) Pp. 213, 1 plate, 233 figures ................. No. 7. The Anculosae of the Alabama River Drainage. By Calvin Goodrich. (1922) Pp. 57, 3plates....................................................... No. 8. The Amphibians and Reptiles of the Sierra Nevada de Santa Marta, Colombia. By Alexander G. Ruthven. (1922) Pp. 69, 13 plates, 2 figures, 1 map ............... No. 9. Notes on American Species of Triacanthagyna and Gynacantha. By E. B. Williamson. (1923) Pp. 67,7 plates ........................ .I................... No. -

A Checklist of North American Odonata, 2021 1 Each Species Entry in the Checklist Is a Paragraph In- Table 2
A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution Dennis R. Paulson and Sidney W. Dunkle 2021 Edition (updated 12 February 2021) A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution 2021 Edition (updated 12 February 2021) Dennis R. Paulson1 and Sidney W. Dunkle2 Originally published as Occasional Paper No. 56, Slater Museum of Natural History, University of Puget Sound, June 1999; completely revised March 2009; updated February 2011, February 2012, October 2016, November 2018, and February 2021. Copyright © 2021 Dennis R. Paulson and Sidney W. Dunkle 2009, 2011, 2012, 2016, 2018, and 2021 editions published by Jim Johnson Cover photo: Male Calopteryx aequabilis, River Jewelwing, from Crab Creek, Grant County, Washington, 27 May 2020. Photo by Netta Smith. 1 1724 NE 98th Street, Seattle, WA 98115 2 8030 Lakeside Parkway, Apt. 8208, Tucson, AZ 85730 ABSTRACT The checklist includes all 471 species of North American Odonata (Canada and the continental United States) considered valid at this time. For each species the original citation, English name, type locality, etymology of both scientific and English names, and approximate distribution are given. Literature citations for original descriptions of all species are given in the appended list of references. INTRODUCTION We publish this as the most comprehensive checklist Table 1. The families of North American Odonata, of all of the North American Odonata. Muttkowski with number of species. (1910) and Needham and Heywood (1929) are long out of date. The Anisoptera and Zygoptera were cov- Family Genera Species ered by Needham, Westfall, and May (2014) and West- fall and May (2006), respectively. -
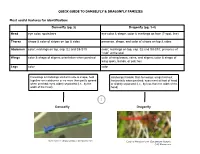
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

A Preliminary Checklist of the Damselflies of Virginia, with Notes on Distribution and Seasonality (Odonata: Zygoptera)
Banisteria, Number 4, 1994 © 1994 by the Virginia Natural History Society A Preliminary Checklist of the Damselflies of Virginia, with Notes on Distribution and Seasonality (Odonata: Zygoptera) Steven M. Roble Division of Natural Heritage Virginia Department of Conservation and Recreation 1500. E. Main Street, Suite 312 Richmond, VA 23219 Virginia has a diverse fauna of aquatic insects, ginia's boundaries occurred in 1862 (R. L. Hoffman, pers. although much additional inventory is needed to fully comm.), subsequent authors (e.g., Muttkowski, 1910; catalog this diversity. Species new to science continue to Needham & Heywood, 1929) failed to account for it in be discovered in the state (e.g., Kondratieff & Kirchner, their range descriptions for several species. Valid Virginia 1994). The aquatic groups treated in the "Insects of records have since been published for all but one (Isch- Virginia" series to date are limited to the true bugs and nura prognata) of these species. several families of beetles and flies (Bobb, 1974; Gladney The following annotated checklist of the state's & Turner, 1969; Matta, 1974, 1976; Michael & Matta, damselfly fauna should be considered as preliminary. I 1977; Pechuman, 1973). Species checklists have been have not conducted an exhaustive search of available compiled for the stoneflies (Kondratieff & Voshell, 1979; collections in preparing this list. In addition to published Kondratieff & Kirchner, 1987), mayflies (Kondratieff & records, my sources are primarily limited to the collection Voshell, 1983), caddisflies (Parker & Voshell, 1981), and of the United States National Museum of Natural dragonflies (Carle, 1978, 1979, 1982) of the state. The History, Washington, D.C. (abbreviated as USNM present contribution is the first attempt to publish a hereafter) and specimens collected statewide from 1988- comprehensive list of the damselfly species known from 1994 by the zoological staff of the Division of Natural Virginia. -

The Dragonflies (Insecta: Odonata) of the Columbia Basin, British Columbia: Field Surveys, Collections Development and Public Education by Robert A
Living Landscapes The Dragonflies (Insecta: Odonata) of the Columbia Basin, British Columbia: Field Surveys, Collections Development and Public Education by Robert A. Cannings, RBCM, Sydney G. Cannings, CDC, and Leah Ramsay, CDC The Dragonflies (Insecta: Odonata) of the Columbia Basin, British Columbia: Field Surveys, Collections Development and Public Education by: Robert A. Cannings, Royal BC Museum Sydney G. Cannings, B.C. Conservation Data Centre Leah Ramsay, B.C. Conservation Data Centre Table of Contents CIP data Acknowledgements Overview of the Project Introduction to the Dragonflies of the Columbia Basin Dragonfly Habitat in the Columbia Basin Biogeography and Faunal Elements Systematic Review of the Fauna Suborder Zygoptera (Damselflies) Family Calopterygidae (Jewelwings) Family Lestidae (Spreadwings) Family Coenagrionidae (Pond Damsels) Suborder Anisoptera (Dragonflies) Family Aeshnidae (Darners) Family Gomphidae (Clubtails) Family Cordulegastridae (Spiketails) Family Macromiidae (Cruisers) Family Corduliidae (Emeralds) Family Libellulidae (Skimmers) The Effects of Human Activity on Dragonfly Populations Recommendations for Future Inventory, Research and Monitoring References Appendix 1: Checklist of Columbia Basin Dragonflies Appendix 2: Columbia Basin Odonata and Their Faunal Elements Appendix 3: Project Participants Species Distribution Maps and Collecting Data Royal British Columbia Museum 1-888-447-7977 1 675 Belleville Street (250) 356-7226 Copyright 2000 Royal British Columbia Museum Victoria, British Columbia http://www.royalbcmuseum.bc.ca -

The Checklist of Montana Dragonflies & Damselflies
About this Checklist deposit the eggs of further generations. This period River Bluet S c Emma’s Dancer NW,SW,SC o Dragonflies and Damselflies belong to the insect of adult activity is called the Flight Season. Following Enallagma anna M J J A S O N Argia emma M J J A S O N order Odonata, which is split into two suborders: each species is a phenogram [ M J J A S O N ], and Anisoptera – Dragonflies and Zygoptera highlighted in red are the months (May – Nov.) when Familiar Bluet NE,SE c – Damselflies. This checklist includes 53 species of one might expect to see that species during the year. Enallagma civile M J J A S O N Dragonflies (Anisoptera) Dragonflies and 29 species of Damselflies which are Tule Bluet S c known to occur within the state of Montana. Each Species Observed through Oct. 2009 Darners Aeshnidae Enallagma carunculatum M J J A S O N species is listed under its family name and genus. Mosaic Darners Aeshna Common and scientific names are current with those Alkali Bluet S u Damselflies (Zygoptera) Black-tipped Darner NW u set by the Checklist Committee of the Dragonfly Enallagma clausum M J J A S O N Society of the Americas. Aeshna tuberculifera M J J A S O N Broad-winged Damsels Calopterygidae Northern Bluet S c Sedge Darner NW,SW u Jewelwings Calopteryx Enallagma annexum M J J A S O N Distribution Aeshna juncea M J J A S O N To the right of each common name, one or more River Jewelwing NW,SW u Boreal Bluet S c of the following regions will be listed to show the Subarctic Darner NW,SW r Calopteryx aequabilis M J J A S O N Enallagma boreale M J J A S O N approximate distribution of the species within the Aeshna subarctica M J J A S O N Marsh Bluet S c state. -

List of Rare, Threatened, and Endangered Animals of Maryland
List of Rare, Threatened, and Endangered Animals of Maryland December 2016 Maryland Wildlife and Heritage Service Natural Heritage Program Larry Hogan, Governor Mark Belton, Secretary Wildlife & Heritage Service Natural Heritage Program Tawes State Office Building, E-1 580 Taylor Avenue Annapolis, MD 21401 410-260-8540 Fax 410-260-8596 dnr.maryland.gov Additional Telephone Contact Information: Toll free in Maryland: 877-620-8DNR ext. 8540 OR Individual unit/program toll-free number Out of state call: 410-260-8540 Text Telephone (TTY) users call via the Maryland Relay The facilities and services of the Maryland Department of Natural Resources are available to all without regard to race, color, religion, sex, sexual orientation, age, national origin or physical or mental disability. This document is available in alternative format upon request from a qualified individual with disability. Cover photo: A mating pair of the Appalachian Jewelwing (Calopteryx angustipennis), a rare damselfly in Maryland. (Photo credit, James McCann) ACKNOWLEDGMENTS The Maryland Department of Natural Resources would like to express sincere appreciation to the many scientists and naturalists who willingly share information and provide their expertise to further our mission of conserving Maryland’s natural heritage. Publication of this list is made possible by taxpayer donations to Maryland’s Chesapeake Bay and Endangered Species Fund. Suggested citation: Maryland Natural Heritage Program. 2016. List of Rare, Threatened, and Endangered Animals of Maryland. Maryland Department of Natural Resources, 580 Taylor Avenue, Annapolis, MD 21401. 03-1272016-633. INTRODUCTION The following list comprises 514 native Maryland animals that are among the least understood, the rarest, and the most in need of conservation efforts. -

Species List Provid- S's). Laysia, July Widely
Odonatological Abstracts 1999 revealed a small but significant decrease in the aver- number of with age spp. present increasing height (16022) STEFFENS, W.P. & W.A. SMITH, 1999. above the ground. Species richness and abundance Status survey for special concern and endangered were greater in larger holes. Similar patterns were dragonflies of Minnesota: population status, inven- observed in 206 natural tree holes. Of7 top predator and recommendations. Minnesota 3 odon. coerulatus tory monitoring spp. (inch taxa), Megaloprepus Dept Natural Resourses (Natural Heritage & Non- larvae were not found in artificial or natural holes — not game Research Program). 56 pp. (Addresses above 7 m. Chemical properties of tree hole water did ditfer with but holes stated). not height, canopy tree for Status determination surveys Ophiogomphus dried out more frequently and were thermally less anomalus,O. susbehcha and Somatochlora hineana stable than midstory and understory holes. Harsh were conducted throughout eastern, central and thermal conditions and higher disturbance frequen- northern USA. Threats these for the decline in rich- Minnesota, to rare cy may be responsible species evaluated and conservation and spp. were popula- ness with height. tion status recommendations for Minnesota Ani- 2000 soptera are presented. Baseline data on other Ani- sopt. in undersurveyed habitats are reported, in- and cludingseveral state records numerouscounty (16024) BASS, D„ 2000. A preliminary study of records. Several Zygoptera collections are also re- aquaticmacroinvertebrates from two springs in the ported alongwith county distribution information, Pontotoc RidgeNature Preserve, Oklahoma. Proc. and recommendations for future odon. and - Univ. surveys Okla. Acad. Sci. 80: 105-109. (Dept Biol., monitoring are offered. -

Appendix 5: Fauna Known to Occur on Fort Drum
Appendix 5: Fauna Known to Occur on Fort Drum LIST OF FAUNA KNOWN TO OCCUR ON FORT DRUM as of January 2017. Federally listed species are noted with FT (Federal Threatened) and FE (Federal Endangered); state listed species are noted with SSC (Species of Special Concern), ST (State Threatened, and SE (State Endangered); introduced species are noted with I (Introduced). INSECT SPECIES Except where otherwise noted all insect and invertebrate taxonomy based on (1) Arnett, R.H. 2000. American Insects: A Handbook of the Insects of North America North of Mexico, 2nd edition, CRC Press, 1024 pp; (2) Marshall, S.A. 2013. Insects: Their Natural History and Diversity, Firefly Books, Buffalo, NY, 732 pp.; (3) Bugguide.net, 2003-2017, http://www.bugguide.net/node/view/15740, Iowa State University. ORDER EPHEMEROPTERA--Mayflies Taxonomy based on (1) Peckarsky, B.L., P.R. Fraissinet, M.A. Penton, and D.J. Conklin Jr. 1990. Freshwater Macroinvertebrates of Northeastern North America. Cornell University Press. 456 pp; (2) Merritt, R.W., K.W. Cummins, and M.B. Berg 2008. An Introduction to the Aquatic Insects of North America, 4th Edition. Kendall Hunt Publishing. 1158 pp. FAMILY LEPTOPHLEBIIDAE—Pronggillled Mayflies FAMILY BAETIDAE—Small Minnow Mayflies Habrophleboides sp. Acentrella sp. Habrophlebia sp. Acerpenna sp. Leptophlebia sp. Baetis sp. Paraleptophlebia sp. Callibaetis sp. Centroptilum sp. FAMILY CAENIDAE—Small Squaregilled Mayflies Diphetor sp. Brachycercus sp. Heterocloeon sp. Caenis sp. Paracloeodes sp. Plauditus sp. FAMILY EPHEMERELLIDAE—Spiny Crawler Procloeon sp. Mayflies Pseudocentroptiloides sp. Caurinella sp. Pseudocloeon sp. Drunela sp. Ephemerella sp. FAMILY METRETOPODIDAE—Cleftfooted Minnow Eurylophella sp. Mayflies Serratella sp. -

Of Calopteryx Aequabilis Say, C. Aequabilis Is Recorded Disjunctus
Four species of Odonata new to British Columbia, Canada 4 R.A. Cannings¹,S.G. Cannings²,L.R. Ramsay³ and G.E. Hutchings 1 Royal British Columbia Museum, 675 Belleville Street, Victoria, British Columbia,V8W 9W2, Canada 2 NatureServe Yukon, Yukon Department of Environment, Box 2703, Whitehorse, Yukon Terri- tory, Y1A 2C6, Canada 3 British Columbia Conservation Data Centre, British Columbia Ministry of Sustainable Resource Management,P.O. Box 9358, Stn. Prov. Govt., Victoria, British Columbia, V8W 9M2, Canada 4 971 Arundel Drive, Victoria, British Columbia,V9A 2C4, Canada Abstract –- Between 1998 and 2000, 5 odon. bia each is recorded from a handful of localities added the list of British Columbia. in mountain and northern spp. were to peatlands. The collection data for one of these, Somato- chlora have been Introduction kennedyi, previously published by R.D. Kenner (2000, J. ent. Soc. Br. Colwnb. Between 1996 and 2003, the Royal British Co- 97: 47-49). The present known distribution, sta- lumbia Museum (RBCM) and the British Co- tus and habitat of Calopteryx aequabilis Say, lumbia Conservation Data Centre (BCCDC) Lestes Somatochlora brev- collaborated in of the Odonata of forcipatus Ramb., surveys sev- icincta Robert and S. forcipata (Scudder) are eral regions of British Columbia (BC), Cana- discussed. C. aequabilis is recorded from only da - the Georgia Depression of the southwest one locality in the extreme S of the province; coast (1996), the Okanagan drainage(1997), the it is a red-listed sp. of management concern. L. Peace River-Fort Nelson region (1997), the Co- forcipatus is common in certain types of rich lumbia-Kootenay region (1998-99) and central fens the it had been overlooked and northwestern BC The across province; (2000-2003). -

The Dragonflies and Damselflies (Odonata) of Canadian Grasslands
231 Chapter 8 The Dragonfl ies and Damselfl ies (Odonata) of Canadian Grasslands Robert A. Cannings Royal British Columbia Museum, 675 Belleville Street Victoria, British Columbia, V8W 9W2 [email protected] Abstract. The Odonata are energetic aerial predators of other insects; the aquatic larvae are voracious predators of invertebrates and small vertebrates. As of 2010, 5,952 species of the order were described worldwide; 211 species are known from Canada. Grasslands across the country support about 59% of the national fauna. A checklist and systematic overview of 124 species in nine families are presented. Species totals in these families are as follows: Calopterygidae, 2; Lestidae, 7; Coenagrionidae, 31; Aeshnidae, 16; Gomphidae, 15; Cordulegastridae, 1; Macromiidae, 2; Corduliidae, 13; and Libellulidae, 37. The geographical ranges of the species are defi ned and summarized; according to the defi nitions herein, 20 species have boreal ranges, 17 are transition species, 12 are Cordilleran, 1 is Pacifi c coastal, 10 are western, 4 are more or less restricted to the Great Plains, 16 have southern ranges, 38 are considered eastern, and 6 are widespread species. A summary of studies on grassland Odonata and recommendations for inventory and taxonomic research are provided. The geographical scope of the Canadian grassland fauna is described briefl y with respect to lotic and lentic habitats in grasslands of the Cordillera, the Great Plains, and southern Ontario. Résumé. Les odonates sont de féroces prédateurs aériens d’autres insectes ; leurs larves aquatiques sont aussi des prédateurs voraces d’autres invertébrés et petits vertébrés. En 2010, 5 952espèces d’odonates avaient été décrites dans le monde.