The Dendritic Complexity and Innervation of Submandibular Neurons in Five Species of Klamkals
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Sympathetic and the Parasympathetic Nervous System
The sympathetic and the parasympathetic nervous system Zsuzsanna Tóth, PhD Institute of Anatomy, Histology and Embryology Semmelweis University The role of the autonomic nervous system Claude Bernard • „milieu intérieur” concept; every organism lives in its internal environment that is constant and independent form the external environment Walter Bradford Cannon homeostasis; • an extension of the “milieu interieur” concept • consistence in an open system requires mechanisms that act to maintain that consistency • steady-state conditions require that any tendency toward change automatically meets with factors that resist that change • regulating systems that determine the homeostatic state : o autonomic nervous system ( sympathetic, parasympathetic, enteral) o endocrine system General structure of the autonomic nervous system craniosacral thoracolumbar Anatomy Neurotransmittersof the gut autonomic nervous system. symp. gangl pregangl. fiber pregangl. postgangl. fiber fiber (PoR) PoR enteral ganglion PoR PoR smooth muscle smooth muscle Kuratani S Development 2009;136:1585-1589 Sympathetic activation: Fight or flight reaction • energy mobilization • preparation for escape, or fight vasoconstriction • generalized Parasympathetic activation: adrenal • energy saving and restoring • „rest and digest” system • more localized vasoconstriction Paravertebral ganglia and the sympathetic chains pars cervicalis superius ganglion medium cervicale stellatum pars vertebrae • from the base of the skull to the caudal end thoracalis thoracalis of the sacrum • paravertebral ganglia (ganglia trunci sympathici) • rami interganglionares pars vertebrae • the two chains fuses at the ganglion impar abdominalis lumbalis sacrum pars pelvina foramen sacralia anteriora ganglion impar Anatomy of the cervical part of the sympathetic trunk superior cervical ganglion • behind the seath of the carotid, fusiform ggl. cervicale superius • IML T1-3 vegetative motoneurons- preganglionic fibers truncus symp. -

Simple Ways to Dissect Ciliary Ganglion for Orbital Anatomical Education
OkajimasDetection Folia Anat. of ciliary Jpn., ganglion94(3): 119–124, for orbit November, anatomy 2017119 Simple ways to dissect ciliary ganglion for orbital anatomical education By Ming ZHOU, Ryoji SUZUKI, Hideo AKASHI, Akimitsu ISHIZAWA, Yoshinori KANATSU, Kodai FUNAKOSHI, Hiroshi ABE Department of Anatomy, Akita University Graduate School of Medicine, Akita, 010-8543 Japan –Received for Publication, September 21, 2017– Key Words: ciliary ganglion, orbit, human anatomy, anatomical education Summary: In the case of anatomical dissection as part of medical education, it is difficult for medical students to find the ciliary ganglion (CG) since it is small and located deeply in the orbit between the optic nerve and the lateral rectus muscle and embedded in the orbital fat. Here, we would like to introduce simple ways to find the CG by 1): tracing the sensory and parasympathetic roots to find the CG from the superior direction above the orbit, 2): transecting and retracting the lateral rectus muscle to visualize the CG from the lateral direction of the orbit, and 3): taking out whole orbital structures first and dissecting to observe the CG. The advantages and disadvantages of these methods are discussed from the standpoint of decreased laboratory time and students as beginners at orbital anatomy. Introduction dissection course for the first time and with limited time. In addition, there are few clear pictures in anatomical The ciliary ganglion (CG) is one of the four para- textbooks showing the morphology of the CG. There are sympathetic ganglia in the head and neck region located some scientific articles concerning how to visualize the behind the eyeball between the optic nerve and the lateral CG, but they are mostly based on the clinical approaches rectus muscle in the apex of the orbit (Siessere et al., rather than based on the anatomical procedure for medical 2008). -

Clinical Anatomy of the Trigeminal Nerve
Clinical Anatomy of Trigeminal through the superior orbital fissure Nerve and courses within the lateral wall of the cavernous sinus on its way The trigeminal nerve is the fifth of to the trigeminal ganglion. the twelve cranial nerves. Often Ophthalmic Nerve is formed by the referred to as "the great sensory union of the frontal nerve, nerve of the head and neck", it is nasociliary nerve, and lacrimal named for its three major sensory nerve. Branches of the ophthalmic branches. The ophthalmic nerve nerve convey sensory information (V1), maxillary nerve (V2), and from the skin of the forehead, mandibular nerve (V3) are literally upper eyelids, and lateral aspects "three twins" carrying information of the nose. about light touch, temperature, • The maxillary nerve (V2) pain, and proprioception from the enters the middle cranial fossa face and scalp to the brainstem. through foramen rotundum and may or may not pass through the • The three branches converge on cavernous sinus en route to the the trigeminal ganglion (also called trigeminal ganglion. Branches of the semilunar ganglion or the maxillary nerve convey sensory gasserian ganglion), which contains information from the lower eyelids, the cell bodies of incoming sensory zygomae, and upper lip. It is nerve fibers. The trigeminal formed by the union of the ganglion is analogous to the dorsal zygomatic nerve and infraorbital root ganglia of the spinal cord, nerve. which contain the cell bodies of • The mandibular nerve (V3) incoming sensory fibers from the enters the middle cranial fossa rest of the body. through foramen ovale, coursing • From the trigeminal ganglion, a directly into the trigeminal single large sensory root enters the ganglion. -

Catecholaminergic Properties of Cholinergic Neurons and Synapses in Adult Rat Ciliary Ganglion
The Journal of Neuroscience, November 1987, 7(11): 35743587 Catecholaminergic Properties of Cholinergic Neurons and Synapses in Adult Rat Ciliary Ganglion Story C. Landis,’ Patrick C. Jackson,l,a John R. Fredieu,l,b and Jean ThibauW ‘Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115, and 2CoIlege de France, Paris, France Parasympathetic neurons of the ciliary ganglion are inner- The developmental mechanisms responsible for these mixed vated by preganglionic cholinergic neurons whose cell bod- transmitter phenotypes and the functional consequences re- ies lie in the brain stem; the ganglion cells in turn provide main to be elucidated. cholinergic innervation to the intrinsic muscles of the eye. Noradrenergic innervation of the iris is supplied by sympa- thetic neurons of the superior cervical ganglion. Using im- The ciliary ganglion is classified as a parasympathetic ganglion munocytochemical and histochemical techniques, we have based on anatomical, biochemical, and pharmacological crite- examined the ciliary ganglion of adult rats for the expression ria. The ganglion lies close to its target tissues, the iris and ciliary of cholinergic and noradrenergic properties. As expected, body; the preganglionic neurons lie in the brain stem (Warwick, the postganglionic ciliary neurons possessed detectable 1954; Loewy et al., 1978; Johnson and Purves, 198 1). In the levels of choline acetyltransferase immunoreactivity (ChAT- cat, the mammal studied most extensively, the ganglion contains IR). Unexpectedly, many ciliary neurons also exhibited im- high levels of ChAT, reflecting enzyme present in both pregan- munoreactivity for tyrosine hydroxylase (TH-IR). Some had glionic terminals and postganglionic perikarya (Buckley et al., dopamine&hydroxylase-like (DBH-IR) immunoreactivity, but 1967). -

Sympathetic Tales: Subdivisons of the Autonomic Nervous System and the Impact of Developmental Studies Uwe Ernsberger* and Hermann Rohrer
Ernsberger and Rohrer Neural Development (2018) 13:20 https://doi.org/10.1186/s13064-018-0117-6 REVIEW Open Access Sympathetic tales: subdivisons of the autonomic nervous system and the impact of developmental studies Uwe Ernsberger* and Hermann Rohrer Abstract Remarkable progress in a range of biomedical disciplines has promoted the understanding of the cellular components of the autonomic nervous system and their differentiation during development to a critical level. Characterization of the gene expression fingerprints of individual neurons and identification of the key regulators of autonomic neuron differentiation enables us to comprehend the development of different sets of autonomic neurons. Their individual functional properties emerge as a consequence of differential gene expression initiated by the action of specific developmental regulators. In this review, we delineate the anatomical and physiological observations that led to the subdivision into sympathetic and parasympathetic domains and analyze how the recent molecular insights melt into and challenge the classical description of the autonomic nervous system. Keywords: Sympathetic, Parasympathetic, Transcription factor, Preganglionic, Postganglionic, Autonomic nervous system, Sacral, Pelvic ganglion, Heart Background interplay of nervous and hormonal control in particular The “great sympathetic”... “was the principal means of mediated by the sympathetic nervous system and the ad- bringing about the sympathies of the body”. With these renal gland in adapting the internal -

Autonomic Nervous System
Autonomic nervous System Regulates activity of: Smooth muscle Cardiac muscle certain glands Autonomic- illusory (convenient)-not under direct control Regulated by: hypothalamus Medulla oblongata Divided in to two subdivisions: Sympathetic Parasympathetic Sympathetic: mobilizes all the resources of body in an emergency Parasympathetic: maintains the normal body functions Complimentary to each other. ANS Activity expressed • Regulation of Blood Pressure • Regulation of Body Temperature • Cardio-respiratory rate • Gastro-intestinal motility • Glandular Secretion Sensations • General – Hunger , Thirst , Nausea • Special -- Smell, taste and visceral pain • Location of ANS in CNS: 1. cerebral hemispheres (limbic system) 2. Brain stem (general visceral nuclei of cranial nerves) 3. Spinal cord (intermediate grey column) ANS Anatomy • Pathway: Two motor neurons 1. In CNS -->Axon-->Autonomic ganglion 2. In Autonomic ganglion-->Axon-->effector organ • Anatomy: Preganglionic neuron--->preganglionic fibre (myelinated axon)--->out of CNS as a part of cranial/spinal nerve--->fibres separate & extend to ANS ganglion-->synapse with postganglionic neuron--->postganglionic fibre (nonmyelinated)-- >effector organ Sympathetic system Components • Pair of ganglionic sympathetic trunk • Communicating rami • Branches • Plexuses • Subsidiary ganglia – collateral , terminal ganglia Sympathetic trunk (lateral ganglia) • Paravertebral in position • Extend from base of skull to coccygeal • Both trunk unite to form – ganglion impar Total Ganglia • Cervical-3 • Thoracic-11 -

Cranial Nerves
Cranial nerves Trigeminal, Facial and Accessory nerves Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology Anatomically, the course of the facial nerve can Facial nerve be divided into two parts: Motor: Innervates the muscles of facial Intracranial – the course of the nerve through expression, the posterior belly of the the cranial cavity, and the cranium itself. digastric, the stylohyoid and the stapedius Extracranial – the course of the nerve outside muscles. the cranium, through the face and neck. Sensory: A small area around the concha of the auricle, EAM Special Sensory: Provides special taste sensation to the anterior 2/3 of the tongue. Parasympathetic: Supplies many of the glands of the head and neck, including: 1- Submandibular and sublingual salivary glands (via the submandibular ganglion/ chorda tympani) 2- Nasal, palatine and pharyngeal mucous glands (via the pterygopalatine ganglion/ greater petrosal) 3- Lacrimal glands (via the pterygopalatine ganglion/ greater petrosal) Dr. Heba Kalbouneh Intracranial course Dr. Heba Kalbouneh The nerve arises in the pons. It begins as two roots; a large motor root, and a small sensory root The two roots travel through the internal acoustic meatus. Here, they are in very close proximity to the inner ear. 7th (motor) 8th Note: The part of the facial nerve that runs between the motor root of facial and vestibulocochlear nerve is sometimes known as the nervus intermedius It contains the sensory and parasympathetic fibers of the facial nerve Carotid plexus Deep petrosal n around ICA Pterygopalatine ganglion Foramen lacerum Facial nerve Nerve of pterygoid canal Internal acoustic meatus Greater petrosal n Geniculate ganglion N to stapedius Chorda tympani Lingual n Stylomastoid foramen Submandibular ganglion Posterior auricular n Parotid gland Stylohyoid Post belly of digastric Kalbouneh Heba Dr. -

Development of the Rat Superior Cervical Ganglion: Initial Stages of Synapse Formation’
0270.6474/0503-0697$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5. No. 3, pp. 697-704 Printed in U.S.A. March 1985 Development of the Rat Superior Cervical Ganglion: Initial Stages of Synapse Formation’ ERIC RUBIN* Department of Physiology and Biophysics, Washington University School of Medicine, St. Louis, Missouri 63110 Abstract some aspects of synaptic organization through the prenatal period. Some of these results have been briefly reported (Rubin, 1982). Synapse formation in the rat superior cervical ganglion has been investigated electrophysiologically and at the ultra- Materials and Methods structural level. Preganglionic axons first enter the superior The procedures for obtaining and isolating fetal rats have been described cervical ganglion between days 12 and 13 of gestation (El2 (Rubin 1985a, b). As in the previous papers, the day of conception is counted to E13), and on El3 a postganglionic response can be evoked as embryonic day zero (EO), and the first postnatal day is termed PO. by preganglionic stimulation. The susceptibility of this re- flectrophysiology. In isolated fetuses, the right superior cervical ganglion sponse to fatigue and to blocking agents indicates that it is was exposed, along with the internal carotid nerve and the cervical sympa- mediated by cholinergic synapses. On E14, the overall thetic trunk (see Rubin, 1985a). The dissection was carried out at room strength of ganglionic innervation arising from different temperature in a standard Ringer’s solution (pH 7.2) of the following com- spinal segments already varies in a pattern resembling that position (in millimolar concentration): NaCI, 137.0; KCI, 4.0; MgCIP, 1.0; found in maturity. -

Nerve Cell Bodies and Small Ganglia in the Connective Tissue Stroma of Human Submandibular Glands
Neuroscience Letters 475 (2010) 53–55 Contents lists available at ScienceDirect Neuroscience Letters journal homepage: www.elsevier.com/locate/neulet Nerve cell bodies and small ganglia in the connective tissue stroma of human submandibular glands Konstantinos I. Tosios a,∗, Michail Nikolakis a, Andreas Christoforos Prigkos a, Smaragda Diamanti a,b, Alexandra Sklavounou a a Department of Oral Pathology, Dental School, National and Kapodistrian University of Athens, 11527 Athens, Greece b Stomatology Clinic, 251 Hellenic Air Force General Hospital, Athens, Greece article info abstract Article history: The objective of the study was to investigate the presence and distribution of nerve cell bodies and Received 13 February 2010 small ganglia in the stroma of human submandibular gland. A retrospective immunohistochemical study Received in revised form 15 March 2010 in 13 human submandibular glands, fixed in neutral buffered formalin and embedded in paraffin wax, Accepted 16 March 2010 was undertaken. Six glands were excised in the course of radical neck dissection for oral squamous cell carcinoma and were disease-free, six showed sialadenitis, and one was involved by tuberculosis. Primary Keywords: antibodies applied were neuron specific enolase, synaptophysin, and glial fibrilliary acidic protein. Neuron Salivary glands specific enolase and synaptophysin positive nerve cell bodies and small ganglia were found in 8/13 and Submandibular gland Nerve tissue 13/13 glands, respectively. They were found in the interlobular connective tissue stroma of human SMG, Ganglion cells in close association to salivary parenchymal cells and blood vessels, and some of them were incorporated in GFAP positive peripheral nerves. To our knowledge, nerve cell bodies and small ganglia have been described only in the connective tissue stroma of autotransplanted human SMG and their functional importance is not clear. -

Morphometric Study of the Ciliary Ganglion and Its Pertinent Intraorbital Procedure L
Folia Morphol. Vol. 79, No. 3, pp. 438–444 DOI: 10.5603/FM.a2019.0112 O R I G I N A L A R T I C L E Copyright © 2020 Via Medica ISSN 0015–5659 journals.viamedica.pl Morphometric study of the ciliary ganglion and its pertinent intraorbital procedure L. Tesapirat1, S. Jariyakosol2, 3, V. Chentanez1 1Department of Anatomy, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand 2Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand 3Ophthalmology Department, King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand [Received: 20 September 2019; Accepted: 12 October 2019] Background: Ciliary ganglion (CG) can be easily injured without notice in many intraorbital procedures. Surgical procedures approaching the lateral side of the orbit are at risk of CG injury which results in transient mydriasis and tonic pupil. This study aims to focus on the morphometric study of the CG which is pertinent to intraoperative procedure. Materials and methods: Forty embalmed cadaveric globes were dissected to ob- serve the location, shape and size of CG, characteristics and number of roots reaching CG, number of short ciliary nerve in the orbit. Distances from CG to posterior end of globe, optic nerve, lateral rectus muscle and its scleral insertion were measured. Results: Ciliary ganglion was located between optic nerve and lateral rectus in every case. Its shape could be oval, round and irregular. Mean width of CG was 2.24 mm and mean length was 3.50 mm. Concerning the roots, all 3 roots were present in 29 (72.5%) cases. Absence of motor root was found in 7 (17.5%) cases. -
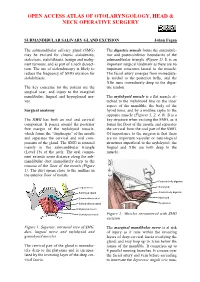
Submandibular Gland Excision
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY SUBMANDIBULAR SALIVARY GLAND EXCISION Johan Fagan The submandibular salivary gland (SMG) The digastric muscle forms the anteroinfe- may be excised for chronic sialadenitis, rior and posteroinferior boundaries of the sialectasis, sialolithiasis, benign and malig- submandibular triangle (Figure 2). It is an nant tumours, and as part of a neck dissect- important surgical landmark as there are no tion. The use of sialendoscopy is likely to important structures lateral to the muscle. reduce the frequency of SMG excision for The facial artery emerges from immediate- sialolithiasis. ly medial to the posterior belly, and the XIIn runs immediately deep to the digas- The key concerns for the patient are the tric tendon. surgical scar, and injury to the marginal mandibular, lingual and hypoglossal ner- The mylohyoid muscle is a flat muscle at- ves. tached to the mylohyoid line on the inner aspect of the mandible, the body of the Surgical anatomy hyoid bone, and by a midline raphe to the opposite muscle (Figures 1, 2, 4, 8). It is a The SMG has both an oral and cervical key structure when excising the SMG, as it component. It passes around the posterior forms the floor of the mouth, and separates free margin of the mylohyoid muscle, the cervical from the oral part of the SMG. which forms the “diaphragm” of the mouth Of importance to the surgeon is that there and separates the cervical and oral com- are no important vascular or neurological ponents of the gland. The SMG is situated structures superficial to the mylohyoid; the mainly in the submandibular triangle lingual and XIIn are both deep to the (Level 1b) of the neck. -

82476025.Pdf
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Neuron, Vol. 22, 253±263, February, 1999, Copyright 1999 by Cell Press Gene Targeting Reveals a Critical Role for Neurturin in the Development and Maintenance of Enteric, Sensory, and Parasympathetic Neurons Robert O. Heuckeroth,1,2 Hideki Enomoto,3 enteric neurons (Hearn et al., 1998; Heuckeroth et al., John R. Grider,6 Judith P. Golden,2,4 1998). Artemin has biological activities that are similar Julie A. Hanke,1,2 Alana Jackman,4 to GDNF and Neurturin in systems where it has been Derek C. Molliver,4,8 Mark E. Bardgett,5 tested (Baloh et al., 1998b). In contrast, Persephin, which William D. Snider,4 Eugene M. Johnson, Jr.,2,4 has similar neurotrophic actions on CNS neurons, does and Jeffrey Milbrandt3,7 not support survival of peripheral neuron populations 1Department of Pediatrics (Heuckeroth et al., 1998; Milbrandt et al., 1998). The 2Department of Molecular Biology and Pharmacology difference in biological activity between Persephin and 3Departments of Pathology and Internal Medicine the other family members in vitro may reflect the differ- 4Department of Neurology ences in GFRa coreceptor specificity for these ligands. 5Department of Psychiatry GFRa1±3 (Jing et al., 1996; Treanor et al., 1996; Baloh Washington University School of Medicine et al., 1997, 1998a; Buj-Bello et al., 1997; Klein et al., St. Louis, Missouri 63110 1997; Widenfalk et al., 1997; Naveilhan et al., 1998; No- 6Departments of Physiology and Medicine moto et al., 1998; Trupp et al., 1998; Worby et al., 1998) Medical College of Virginia and (at least in avian systems) GFRa4 (Thompson et of Virginia Commonwealth University al., 1998) comprise a family of high-affinity GPI-linked Richmond, Virginia 23298 coreceptors that are required for activation of the Ret kinase.