Bayer Aktiengesellschaft
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Medicaid) Enhanced Care Plus (HARP) 2021 Drug Formulary Emblemhealth Enhanced Care and Enhanced Care Plus Formulary 2021 Formulary (List of Covered Drugs
Enhanced Care (Medicaid) Enhanced Care Plus (HARP) 2021 Drug Formulary EmblemHealth Enhanced Care and Enhanced Care Plus Formulary 2021 Formulary (List of Covered Drugs) PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT some of the drugs covered by your plan. When this drug list (formulary) refers to “we,” “us,” or “our,” it means EmblemHealth. When it refers to “plan” or “our plan,” it means EmblemHealth Enhanced Care (Medicaid) or Enhanced Care Plus (HARP). This document includes a list of the drugs (formulary) for our plan which is current as of 10/1/2021. For a complete updated formulary, please call Customer Service at 888-447-7364 (TTY: 711). Our hours are 8 am to 6 pm, Monday through Friday and 10 am to 1 pm, Saturday and Sunday. A representative will be happy to help. You can also check our website at emblemhealth.com. You must use network pharmacies to use your prescription drug benefit. The formulary and pharmacy network may change. Effective Oct. 1, 2021, New York State Medicaid will implement a single statewide Medication Assisted Treatment (MAT) formulary for opioid dependence agents and opioid antagonists. i EMBLEMHEALTH ENHANCED CARE AND ENHANCED CARE PLUS FORMULARY What is the EmblemHealth Enhanced Care and Enhanced Care Plus Formulary? A formulary is a list of covered drugs selected by our plan with help from a team of health care professionals. It represents the prescription therapies believed to be a necessary part of a quality treatment program. Our plan will cover the drugs listed in our formulary as long as the drug is medically necessary, the prescription is filled at a plan network pharmacy, and other plan rules are followed. -
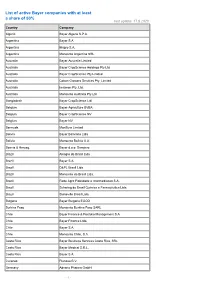
List of Active Bayer Companies with at Least a Share of 50% Last Update: 17.8.2020 Country Company
List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Algeria Bayer Algerie S.P.A. Argentina Bayer S.A. Argentina Biagro S.A. Argentina Monsanto Argentina SRL Australia Bayer Australia Limited Australia Bayer CropScience Holdings Pty Ltd Australia Bayer CropScience Pty Limited Australia Cotton Growers Services Pty. Limited Australia Imaxeon Pty. Ltd. Australia Monsanto Australia Pty Ltd Bangladesh Bayer CropScience Ltd. Belgium Bayer Agriculture BVBA Belgium Bayer CropScience NV Belgium Bayer NV Bermuda MonSure Limited Bolivia Bayer Boliviana Ltda Bolivia Monsanto Bolivia S.A. Bosnia & Herzeg. Bayer d.o.o. Sarajevo Brazil Alkagro do Brasil Ltda Brazil Bayer S.A. Brazil D&PL Brasil Ltda Brazil Monsanto do Brasil Ltda. Brazil Rede Agro Fidelidade e Intermediacao S.A. Brazil Schering do Brasil Química e Farmacêutica Ltda. Brazil Stoneville Brasil Ltda. Bulgaria Bayer Bulgaria EOOD Burkina Faso Monsanto Burkina Faso SARL Chile Bayer Finance & Portfolio Management S.A. Chile Bayer Finance Ltda. Chile Bayer S.A. Chile Monsanto Chile, S.A. Costa Rica Bayer Business Services Costa Rica, SRL Costa Rica Bayer Medical S.R.L. Costa Rica Bayer S.A. Curacao Pianosa B.V. Germany Adverio Pharma GmbH - 1 - List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Germany AgrEvo Verwaltungsgesellschaft mbH Germany Alcafleu Management GmbH & Co. KG Germany BGI Deutschland GmbH Germany Bayer 04 Immobilien GmbH Germany Bayer 04 Leverkusen Fußball GmbH Germany Bayer 04 Leverkusen -

Steven Sanders, Et Al. V. Bayer (AG) Aktiengesellschaft, Et Al. 03-CV-1546-Second Consolidated Amended Complaint
Q,^.IGiNAL &&,iN 2i 4- L UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF NEW YORK -_DOF x Consolidated Civil Action IN RE BAYER AG SECURITIES No. 03 CV 1546 (WHP) LITIGATION Class Action x 71 :n = SECOND CONSOLIDATED AMENDED COMPLAINT Jury Trial Demand MILBERG WEISS BERSHAD & SCHULMAN LLP Melvyn I. Weiss (MW-1392) Michael C . Spencer (MS-8874) Lee A. Weiss (LW-1130) Jennifer Sclar (JS-7313) One Pennsylvania Plaza New York, NY 10119 (212) 594-5300 Attorneys for Lead Plaintiff January 14, 2005 i • ' Table of Contents Page SUMMARY OF CLAIMS ...............................................................................................................2 JURISDICTION AND VENUE ...................................................................................................... 8 A. General Jurisdiction and Venue ............................................................................... 8 B. Subject Matter Jurisdiction Over Claims of Foreign Purchasers on Foreign Exchanges ................................................................................................................ 8 PARTIES .......................................................................................................................................12 RELEVANT EVENTS ..................................................................................................................14 A. Background of the Statin Market ...........................................................................14 B. FDA Approval and Introduction of Baycol; Early Safety Warnings -

Drug Formulary 2019
2019 PREHOSPITAL FORMULARY EL DORADO COUNTY EMERGENCY MEDICAL SERVICES AGENCY 2900 Fair Lane Court Placerville, CA 95667 17th Edition Effective: July 1, 2019 TABLE OF CONTENTS Acetaminophen ................................................................................................................. 3 Activated Charcoal ........................................................................................................... 4 Adenocard ........................................................................................................................... 5 Albuterol Sulfate .................................................................................................................. 6 Amiodarone ......................................................................................................................... 7 Aspirin ..................................................................................................................................... 9 Atropine Sulfate................................................................................................................. 10 Atrovent (Duoneb) ........................................................................................................... 12 Calcium Chloride .............................................................................................................. 13 Dextrose 50% in Water ..................................................................................................... 14 Dextrose 10% in 100cc NS…………………………………………………………… 14 Diphenhydramine -

Notes to the Consolidated Financial Statements of the Bayer Group
Five-Year Summary Bayer Annual Report 2018 Five-Year Summary 2014 2015 2016 2017 2018 Bayer Group (€ million) Sales 41,339 46,085 34,943 35,015 39,586 EBITDA1 8,315 9,573 8,801 8,563 10,266 1 EBITDA before special items 8,685 10,256 9,318 9,288 9,547 1 EBITDA margin before special items (in %) 21.0 22.3 26.7 26.5 24.1 EBIT1 5,395 6,241 5,738 5,903 3,914 1 EBIT before special items 5,833 7,060 6,826 7,130 6,480 Income before income taxes 4,414 5,236 4,773 4,577 2,318 Net income (from continuing and discontinued operations) 3,426 4,110 4,531 7,336 1,695 1 Earnings per share (from continuing and discontinued operations) (€) 4.14 4.97 5.44 8.29 1.80 1 Core earnings per share (from continuing operations) (€) 5.89 6.82 6.67 6.64 5.94 Net cash provided by operating activities (from continuing and discontinued operations) 5,810 6,890 9,089 8,134 7,917 Net financial debt 19,612 17,449 11,778 3,595 35,679 2 Capital expenditures (as per segment table) 2,484 2,554 2,627 2,418 2,564 Bayer AG Total dividend payment 1,861 2,067 2,233 2,402 2,611 Dividend per share (€) 2.25 2.50 2.70 2.80 2.80 Innovation Research and development expenses (€ million) 3,537 4,274 4,405 4,504 5,246 Ratio of R&D expenses to sales – Pharmaceuticals (%) 15.6 16.0 16.7 16.2 15.5 Ratio of R&D expenses to sales – Crop Science (%) 10.3 10.7 11.7 11.7 13.0 Employees in research and development3 13,900 14,753 14,213 14,041 17,275 Employees 3 Number of employees (Dec. -

Glaxosmithkline Aims to Divest Many OTC Brands
OTC11-02-11p1&28FIN.qxd 8/2/11 11:52 Page 1 11 February 2011 COMPANY NEWS 3 GlaxoSmithKline aims to Valeant expands in CEE 3 with PharmaSwiss deal J&J loses sales of US$1.1bn 4 Boots faces up to tough market 5 divest many OTC brands Key brands boost Novartis OTC sales 6 GSK consumer races ahead 7 laxoSmithKline intends to divest non- to divest the non-core brands as soon as pos- Taisho holds steady in weak market 8 Gcore OTC brands with combined an- sible – hopefully before the end of the year – Vicks shipments lift OTC at P&G 9 nual sales of £500 million (C593 million). depending on buyer interest. Perrigo plans spring Allegra launch 10 Chief executive officer Andrew Witty said The announcement came as GlaxoSmith- Consumer Care grows at Merck & Co 11 the move would enable the company’s Con- Kline reported a 7% rise – 5% at constant ex- Omega reports strong quarter 12 sumer Healthcare division to focus more effec- change rates – in 2010 sales at the Consumer Winter woes hit Boiron 13 tively on its “priority or global” brands as well Healthcare division to £5.01 billion. Sales in Pfizer wants Consumer 14 as its operations in emerging markets. Consumer Healthcare’s OTC Medicines busi- to prove worth A spokesperson for GlaxoSmithKline told ness grew by 5% – 3% at constant exchange OTC bulletin that the company was currently rates – to £2.46 billion. GENERAL NEWS 15 compiling the lists of priority and non-core OTC brands, and hoped to release more details Recent deals with Meda and Valeant Perrigo faces lawsuit over 15 in the second quarter of the year. -

Annual Report 1998 Expertise with Responsibility
Annual Report 1998 Expertise with Responsibility Bayer is a diversified, international chemicals and health care group. We offer our customers a wide variety of products and services in areas ranging from pharmaceuticals and crop protection to plastics, specialty chemicals and imaging technologies. Bayer is research-based and aims for technological leadership in its core activities. Our goals are to steadily increase corporate value and generate a high value added for the benefit of our stockholders, our employees and the community in every country in which we operate. We believe that our technical and commercial expertise involves a responsibility to work for the common good and contribute to sustainable development. Bayer: Success through Expertise with Responsibility. Bayer Key Data Bayer Group 1998 1997 Change in % Sales DM million 54,884 55,005 – 0.2 Operating result DM million 6,151 6,018 + 2.2 Income before income taxes DM million 5,336 5,108 + 4.5 Net income DM million 3,157 2,941 + 7.3 Gross cash flow DM million 6,602 6,480 + 1.9 Stockholders’ equity DM million 24,991 23,923 + 4.5 Total assets DM million 57,216 54,170 + 5.6 Capital expenditures DM million 5,165 4,559 + 13.3 Employees at year end 145,100 144,600 + 0.3 Personnel expenses DM million 15,854 15,442 + 2.7 Research and development expenses DM million 3,920 3,878 + 1.1 Bayer AG 1998 1997 Change in % Total dividend payment DM million 1,461 1,388 + 5.3 Dividend per share DM 2.00 1.90 + 5.3 Tax credit DM 0.65 0.81 - 19.8 Published by Bayer AG, 51368 Leverkusen, This complete version of the Annual Report Germany is published in English and German. -

Bayer Annual Report 2013 Bayer Annual Report 2013 11 for a Better Life for a Better Life
» COVER PICTURE Annual Report 2013 Augmented Version ABOUT THE » Bayer: Science For A Better Life INTEGRATED REPORT » Key Data This year’s Annual Report combinesourfinancialand » Chairman’s Letter our sustainability reporting forthefirsttime.Onpage » FOR A BETTER LIFE 28-29youcanfindfurther » Fighting cancer 10 information about this report » Safeguarding nutrition 16 andlearnhowtouseit. » Conserving resources 22 » TO OUR STOCKHOLDERS » Executive Council 30 » Report of the Supervisory Board 32 » Investor Information 37 01 » COMBINED MANAGEMENT REPORT OF THE BAYER GROUP AND BAYER AG 45 Report on Economic Position 151 » Overview of Sales, Earnings and Financial Position 152 » Business Development by Subgroup, Segment and Region 156 » Earnings; Asset and Financial Position of the Bayer Group 170 » Earnings; Asset and Financial Position of Bayer AG 181 02 » CONSOLIDATED FINANCIAL STATEMENTS OF THE BAYER GROUP 227 » RESPONSIBILITY STATEMENT 330 » INDEPENDENT AUDITORS’ REPORT 331 » INDEPENDENT ASSURANCE REPORT 333 » For direct access 03 FURTHER INFORMATION 336 to a chapter, simply click on its name. » Financial Calendar » Masthead, Disclaimer » TABLE OF CONTENTS Bayer is a global enterprise with core competencies in the fields of health care, agriculture and high-tech polymer materials. As an innovation company, we set trends in research- intensive areas. Our products and services are designed to benefit people and improve their quality of life. At the same time we aim to create value through innovation, growth and high earning power. We are committed to the principles of sustainable development and to our social and ethical responsi- bilities as a corporate citizen. -

OTC Brand Name Extensions Joseph A
University of the Pacific Scholarly Commons School of Pharmacy and Health Sciences Faculty Thomas J. Long School of Pharmacy and Health Articles Sciences 6-1-2004 OTC Brand Name Extensions Joseph A. Woelfel University of the Pacific, [email protected] Follow this and additional works at: https://scholarlycommons.pacific.edu/phs-facarticles Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Woelfel, J. A. (2004). OTC Brand Name Extensions. Pharmacist’s Letter & Prescriber’s Letter, 20(6), 1–5. https://scholarlycommons.pacific.edu/phs-facarticles/72 This Article is brought to you for free and open access by the Thomas J. Long School of Pharmacy and Health Sciences at Scholarly Commons. It has been accepted for inclusion in School of Pharmacy and Health Sciences Faculty Articles by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. ® Detail-Document #200613 −This Detail-Document accompanies the related article published in− ® PHARMACIST’S LETTER / PRESCRIBER’S LETTER June 2004 ~ Volume 20 ~ Number 200613 OTC Brand Name Extensions Some Selected Expected Primary Actual Primary Ingredients Nonprescription Ingredient Product Lines Examples of U.S. Brand Name Extensions Alka-Seltzer4,5 Aspirin There are many different Alka-Seltzer products. Alka-Seltzer Original, Lemon Lime, and Extra Strength all contain aspirin. Alka-Seltzer Morning Relief contains aspirin plus caffeine. Alka-Seltzer PM contains aspirin plus diphenhydramine. The following products contain acetaminophen with other ingredients but no aspirin: Alka-Seltzer Plus Cold Effervescent Tablets and Liqui-Gels, Alka-Seltzer Plus Cold & Sinus, Alka-Seltzer Plus Night-Time Cold Effervescent Tablets and Liqui-Gels, Alka-Seltzer Plus Cold and Cough Liqui-Gels, and Alka-Seltzer Plus Nose and Throat. -

4:17-Cv-00865-AGF Doc
Case: 4:17-cv-00865-AGF Doc. #: 40 Filed: 07/14/17 Page: 1 of 10 PageID #: <pageID> UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MISSOURI EASTERN DIVISION LAVETA JORDAN, et al., ) ) Plaintiffs, ) ) vs. ) Case No. 4:17-CV-865 (CEJ) ) BAYER CORP., et al., ) ) Defendants. ) MEMORANDUM AND ORDER This matter is before the Court on defendants’ motion to dismiss pursuant to Federal Rules of Civil Procedure 8, 9(b), 12(b)(2), and 12(b)(6), and defendants’ motion to sever. Also before the Court are plaintiffs’ motions to remand and stay this action. The issues are fully briefed. I. Background On January 19, 2017, the plaintiffs initiated this action in the Circuit Court for the City of St. Louis, Missouri to recover damages for injuries they allegedly sustained as a result of using Essure, a medical device manufactured and sold by the defendants. In the complaint, plaintiffs assert claims of (1) negligence, (2), negligence per se, (3) negligent misrepresentation, (4) strict liability for failure to warn and manufacturing defects, (5) fraud, (6) constructive fraud, (7) fraudulent concealment, (8) breach of express and implied warranties, (9) violations of assorted state consumer protection laws, (10) Missouri products liability under Mo. Rev. Stat. § 537.760, (11) violation of the Missouri Merchandising Practices Act under Mo. Rev. Stat. § 407.020, and (12) gross negligence. Case: 4:17-cv-00865-AGF Doc. #: 40 Filed: 07/14/17 Page: 2 of 10 PageID #: <pageID> Of the 94 plaintiffs, seven are citizens of Missouri. One plaintiff is an Illinois citizen who allegedly had the device implanted in Missouri. -

Disclosure Information
Disclosure of Financial Relationships with Commercial Interests Disclosure Policy Baylor College of Medicine (BCM) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education (CME) for physicians. BCM is committed to sponsoring CME activities that are scientifically based, accurate, current, and objectively presented. In accordance with the ACCME Standards for Commercial Support, BCM has implemented a mechanism requiring everyone in a position to control the content of an educational activity (i.e., directors, planning committee members, faculty) to disclose any relevant financial relationships with commercial interests (drug/device companies) and manage/resolve any conflicts of interest prior to the activity. Individuals must disclose to participants the existence or non-existence of financial relationships: 1) at the time of the activity or within 12 months prior; and 2) of their spouses/partners. In addition, BCM has requested activity faculty/presenters to disclose to participants any unlabeled use or investigational use of pharmaceutical/device products; to use scientific or generic names (not trade names) in referring to products; and, if necessary to use a trade name, to use the names of similar products or those within a class. Faculty/presenters have also been requested to adhere to the ACCME’s validation of clinical content statements. BCM does not view the existence of financial relationships with commercial interests as implying bias or decreasing the value of a presentation. It is up to participants to determine whether the relationships influence the activity faculty with regard to exposition or conclusions. If at any time during this activity you feel that there has been commercial/promotional bias, notify the Activity Director or Activity Coordinator. -

RESTRICTED Bayer Annual Report 2020 Five-Year Summary 2
Bayer-Geschäftsbericht 2018 A Zusammengefasster Lagebericht 1 Fehler! Kein Text mit angegebener Formatvorlage im Dokument. Annual Report RESTRICTED Bayer Annual Report 2020 Five-Year Summary 2 Five-Year Summary € million 2016 2017 2018 2019 2020 Bayer Group financial KPIs Sales 34,943 35,015 36,742 43,545 41,400 EBITDA1 8,801 8,563 9,695 9,529 (2,910) EBITDA before special items1 9,318 9,288 8,969 11,474 11,461 EBITDA margin before special items1 26.7% 26.5% 24.4% 26.3% 27.7% EBIT1 5,738 5,903 3,454 4,162 (16,169) EBIT before special items1 6,826 7,130 6,013 6,975 7,095 Income before income taxes 4,773 4,577 1,886 2,853 (17,250) Net income (from continuing and discontinued operations) 4,531 7,336 1,695 4,091 (10,495) Earnings per share (from continuing and discontinued operations) (€)1 5.44 8.29 1.80 4.17 (10.68) Core earnings per share (from continuing operations) (€)1 6.67 6.64 5.60 6.38 6.39 Free cash flow 5,806 5,202 4,652 4,214 1,343 Net financial debt 11,778 3,595 35,679 34,068 30,041 Capital expenditures (newly capitalized) 2,627 2,418 2,368 2,920 3,138 Return on Capital Employed (ROCE) (%) 10.3 10.8 4.0 3.7 – 16,5 Bayer AG Total dividend payment 2,233 2,402 2,611 2,751 1,965 Dividend per share (€) 2.70 2.80 2.80 2.80 2.00 Bayer Group nonfinancial KPIs2 Number of smallholder farmers in LMICs who have received support (million) – – – 42 45 Number of women in LMICs who have gained access to modern contraception (million) – – – 38 40 Number of people in underserved communities whose self-care needs have been supported by Bayer interventions