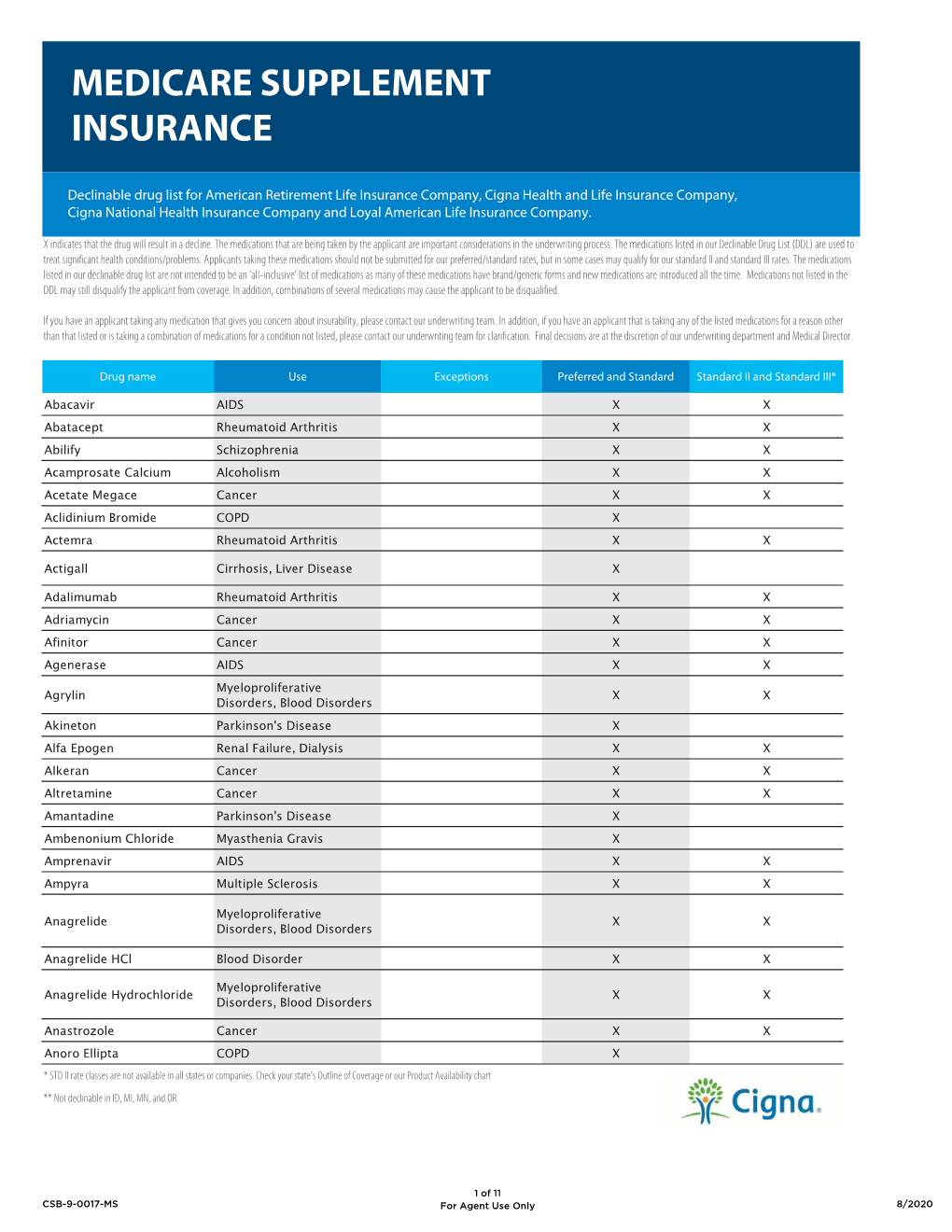
Declinable Drug List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Medicinal Chemistry Unit
JAIPUR COLLEGE OF PHARMACY, JAIPUR B.PHARMACY, SECOND YEAR, FOURTH SEMESTER MEDICINAL CHEMISTRY-I Prepared by: Mrs. Nisha Dhir UNIT-III Cholinergic and Anticholinergic Drug The nervous system is divided into the somatic nervous system, which controls organs under voluntary control (mainly muscles) and the autonomic nervous system (ANS) which regulates individual organ function and homeostasis, and for the most part is not subject to voluntary control. The autonomic nervous system is also known as the visceral or automatic system. The ANS is predominantly an efferent system transmitting impulses from the central nervous system (CNS) to peripheral organ systems. The autonomic nervous system consists of sensory neurons and motor neurons that innervates between the central nervous system (especially the hypothalamus and medulla oblongata) and various internal organs such as the : heart, lungs, viscera, glands (both exocrine and endocrine). Thus it is responsible for monitoring conditions in the internal environment and bringing about appropriate changes in them. The ANS is divided into two separate divisions called the parasympathetic and sympathetic systems, on the basis of anatomical and functional differences. Both of these systems consist of myelinated preganglionic fibres which make synaptic connections with unmyelinated postganglionic fibres, and it is these which then innervate the effector organ. These synapses usually occur in clusters called ganglia. The main nerves of the parasympathetic system are the tenth cranial nerve, the vagus nerve, which originate in the medulla oblongata. Other preganglionic parasympathetic neurons also extend from the brain as well as from the lower tip of the spinal cord. Each preganglionic parasympathetic neuron synapses with just a few postganglionic neurons, which are located near or in the effector organ, a muscle or major gland. -

HMSA DRUG FORMULARY - Listed Alphabetically by GENERIC Name Rev
HMSA DRUG FORMULARY - Listed alphabetically by GENERIC name Rev. 10/1/04 - Page 1 Ther. Generic Brand Select Select Choice Choice QUEST QUEST- CCS Child- Categ. Net ren's Code Code Footnote Code Footnote G = Generic. P = Preferred. O = Other brand. X = Covered. If a number appears next to alpha code, see last page for footnote text. 17 abacavir sulfate Ziagen P O X 17 abacavir/ lamivudine/ zidovudine Trizivir P O X 141 acarbose Precose P P X 50 acebutolol -- G G 50 acebutolol Sectral O O 65 acetaminophen -- X 40 acetazolamide -- G G X 40 acetazolamide Diamox O O 107 acetazolamide -- G G X 107 acetazolamide Diamox O O 40 acetazolamide, extended release Diamox Sequels P O X 239 acetic acid in aluminum acetate -- G G X 239 acetic acid in aluminum acetate Domeboro Otic O O 238 acetic acid, otic -- G G X 238 acetic acid, otic VoSol O O 238 acetic acid/ hydrocortisone, otic -- G G X 238 acetic acid/ hydrocortisone, otic VosSol HC O O 144 acetohexamide -- G G X 144 acetohexamide Dymelor O O 267 acetohydroxamic acid Lithostat O O 99 acetylcarbromal Paxarel O O 198 acetylcysteine -- G G X 198 acetylcysteine Mucomyst O O 243 acitretin Soriatane P O X 255 aclometasone dipropionate 0.05%, cream/ ointment Aclovate O O 173 activated charcoal -- X 22 acyclovir -- G G X 22 acyclovir Zovirax O O 248 acyclovir ointment Zovirax P O X 240 adapalene Differin P 10 O 10 X 10 24 albendazole Albenza O O 188 albuterol -- G G X 188 albuterol Proventil O O 188 albuterol Ventolin O O 189 albuterol -- G G X 189 albuterol Proventil P O X 189 albuterol Proventil HFA O O 189 albuterol Ventolin P O X 189 albuterol Ventolin HFA O O 188 albuterol, extended release -- G G X 188 albuterol, extended release Volmax O O 189 albuterol/ ipratropium Combivent P O X HMSA DRUG FORMULARY - Listed alphabetically by GENERIC name Rev. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0159073 A1 De Juan Et Al
US 20110159073A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0159073 A1 de Juan et al. (43) Pub. Date: Jun. 30, 2011 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA A6F 2/00 (2006.01) (US); Signe E. Varner, Los (52) U.S. Cl. ........................................................ 424/427 Angeles, CA (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Featured is a method for instilling one or more bioactive (21) Appl. No.: 12/981,038 agents into ocular tissue within an eye of a patient for the treatment of an ocular condition, the method comprising con (22) Filed: Dec. 29, 2010 currently using at least two of the following bioactive agent delivery methods (A)-(C): (A) implanting a Sustained release Related U.S. Application Data delivery device comprising one or more bioactive agents in a (63) Continuation of application No. 1 1/175,850, filed on posterior region of the eye so that it delivers the one or more Jul. 5, 2005, now abandoned. bioactive agents into the vitreous humor of the eye; (B) instill ing (e.g., injecting or implanting) one or more bioactive (60) Provisional application No. 60/585,236, filed on Jul. 2, agents Subretinally; and (C) instilling (e.g., injecting or deliv 2004, provisional application No. 60/669,701, filed on ering by ocular iontophoresis) one or more bioactive agents Apr. 8, 2005. into the vitreous humor of the eye. Patent Application Publication Jun. 30, 2011 Sheet 1 of 22 US 2011/O159073 A1 Patent Application Publication Jun. -

Mytelase (Ambenonium Chloride) Tablets Label
NDA 010155/S-022 NDA 010155/ S-023 FDA Approved Labeling Text dated 11/10/2011 Page 1 MYTELASE® AMBENONIUM CHLORIDE DESCRIPTION MYTELASE, brand of ambenonium chloride, is [Oxalylbis (iminoethylene)] bis[(o chlorobenzyl) diethylammonium] dichloride, a white crystalline powder, soluble in water to 20 percent (w/v). Inactive Ingredients: Acacia, Dibasic Calcium Phosphate, Gelatin, Lactose, Magnesium Stearate, Starch, Sucrose. CLINICAL PHARMACOLOGY The compound is a cholinesterase inhibitor with all the pharmacologic actions of acetylcholine, both the muscarinic and nicotinic types. Cholinesterase inactivates acetylcholine. Like neostigmine, MYTELASE suppresses cholinesterase but has the advantage of longer duration of action and fewer side effects on the gastrointestinal tract. The longer duration of action also results in more even strength, better endurance, and greater residual effect during the night and on awakening than is produced by shorter-acting anticholinesterase compounds. INDICATION AND USAGE This drug is indicated for the treatment of myasthenia gravis. CONTRAINDICATIONS Routine administration of atropine with MYTELASE is contraindicated since belladonna derivatives may suppress the parasympathomimetic (muscarinic) symptoms of excessive gastrointestinal stimulation, leaving only the more serious symptoms of fasciculation and paralysis of voluntary muscles as signs of overdosage. MYTELASE should not be administered to patients receiving mecamylamine, or any other ganglionic blocking agents. MYTELASE should also not be administered to patients with a known hypersensitivity to ambenonium chloride or any other ingredients of MYTELASE. WARNINGS Because this drug has a more prolonged action than other antimyasthenic drugs, simultaneous administration with other cholinergics is contraindicated except under strict medical supervision. The overlap in duration of action of several drugs complicates dosage schedules. -

Myasthenia Gravis
Postgrad Med J: first published as 10.1136/pgmj.41.476.356 on 1 June 1965. Downloaded from POSTGRAD. MED. J. (1965), 41, 356 MYASTHENIA GRAVIS I. T. DRAPER, M.R.C.P.E. Neurological Unit, Northern General Hospital, Edinburgh. Definition immune mechanism has been postulated as an MYASTHENIA GRAVIS is a disorder of neuro- atiological factor in the causation of these muscular conduction. It is characterised disorders and it may be that the myasthenic clinically by an excessive degree of weakness patient is genetically predisposed to his disease which develops in the affected muscles during (Simpson, 1960). sustained or repeated contraction. In the early stages of the illness, at least, the strength is Onset partially or fully restored by rest or the The onset of the disease is commonly in- administration of anticholinesterase drugs in sidious, and may at first be neglected by the the appropriate dosage. patient. In such circumstances it is difficult Natural History to establish any precipitating factor. In a Incidence and at onset large number of cases, however, the first age recognised symptoms come on with dramatic The incidence of the disease in the total suddenness. In such a case the precipitating population has been estimated between 1 in factor is more readily identified. This can becopyright. 40,000 (Garland and Clark, 1956) and 1 in a physical or emotional shock, and one may 15 to 20,000 (Eaton, 1958), and it is found be tempted to diagnose hysteria, particularly more commonly in women, in a proportion if the patient is examined after a period of rest 3 to 2 (Schwab and Leland, 1953; Ferguson, when the symptoms and signs have disappeared. -

Antimyasthenics
Antimyasthenics This chapter includes those drugs used for their their reaction to an anticholinesterase. Intravenous edro- • Plasma exchange provides a dramatic but short-lived anticholinesterase action in the treatment of myasthe- phonium preceded by atropine (Tensilon test) is the most improvement and is useful as a short-term measure in nia gravis and related neuromuscular disorders. Other commonly used anticholinesterase test because of its rapid myasthenic crisis to improve ill patients while other groups of drugs playing an important role in the man- onset and short duration of action. Severe adverse effects therapies take effect, but there is no evidence that repeat- agement of myasthenia are the corticosteroids (p.1490) can occasionally occur so testing should only be undertak- ed plasma exchange combined with immunosuppres- en when facilities for endotracheal intubation and control- sion is superior to immunosuppression alone. A similar and some drugs with immunosuppressant actions dis- led ventilation are immediately available. A positive result short-term benefit has been seen from the use of high- cussed in the chapters on Antineoplastics (p.635) and is considered to be a rapid but temporary increase in mus- dose intravenous normal immunoglobulins; however, a Immunosuppressants (p.1810). cle strength. Repetitive nerve stimulation is also used as a systematic review considered further study to be war- diagnostic test but, like the anticholinesterase test, is not ranted. Eaton-Lambert myasthenic syndrome specific for myasthenia gravis. Computed tomography or • Thymectomy may be offered to all patients sufficiently Eaton-Lambert myasthenic syndrome is a rare auto-im- magnetic resonance imaging may be used to detect thymo- fit to undergo surgery unless they have minimal symp- mune disease of the neuromuscular junction.