GFS Organics Modern Acetylene Chemistry for Research and Applied Technology ORDERING INFORMATION
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Investigation of Base-Free Copper-Catalysed Azide–Alkyne Click Cycloadditions (Cuaac) in Natural Deep Eutectic Solvents As Green and Catalytic Reaction Media
Investigation of Base-free Copper-Catalysed Azide–Alkyne Click Cycloadditions (CuAAc) in Natural Deep Eutectic Solvents as Green and Catalytic Reaction Media Salvatore V. Giofrè,1* Matteo Tiecco,2* Angelo Ferlazzo,3 Roberto Romeo,1 Gianluca Ciancaleoni,4 Raimondo Germani2 and Daniela Iannazzo3 1. Dipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università di Messina, Viale Annunziata, I-98168 Messina, Italy. 2. Dipartimento di Chimica, Biologia e Biotecnologie, Università di Perugia, via Elce di Sotto 8, I- 06123 Perugia, Italy. 3. Dipartimento di Ingegneria, Università of Messina, Contrada Di Dio, I-98166 Messina, Italy 4. Dipartimento di Chimica e Chimica Industriale (DCCI), Università di Pisa, Via Giuseppe Moruzzi, 13, I-56124 Pisa, Italy. * Corresponding authors Email addresses: [email protected] (Salvatore V. Giofrè); [email protected] (Matteo Tiecco). ABSTRACT The click cycloaddition reaction of azides and alkynes affording 1,2,3-triazoles is a transformation widely used to obtain relevant products in chemical biology, medicinal chemistry, materials science and other fields. In this work, a set of Natural Deep Eutectic Solvents (NADESs) as “active” reaction media has been investigated in the copper-catalysed azide–alkyne cycloaddition reactions (CuAAc). The use of these green liquids as green and catalytic solvents has shown to improve the reaction effectiveness, giving excellent yields. The NADESs proved to be “active” in this transformation for the absence of added bases in all the performed reactions and in several cases for their reducing capabilities. The results were rationalized by DFT calculations which demonstrated the involvement of H-bonds between DESs and alkynes as well as a stabilization of copper catalytic intermediates. -

14.8 Organic Synthesis Using Alkynes
14_BRCLoudon_pgs4-2.qxd 11/26/08 9:04 AM Page 666 666 CHAPTER 14 • THE CHEMISTRY OF ALKYNES The reaction of acetylenic anions with alkyl halides or sulfonates is important because it is another method of carbon–carbon bond formation. Let’s review the methods covered so far: 1. cyclopropane formation by the addition of carbenes to alkenes (Sec. 9.8) 2. reaction of Grignard reagents with ethylene oxide and lithium organocuprate reagents with epoxides (Sec. 11.4C) 3. reaction of acetylenic anions with alkyl halides or sulfonates (this section) PROBLEMS 14.18 Give the structures of the products in each of the following reactions. (a) ' _ CH3CC Na| CH3CH2 I 3 + L (b) ' _ butyl tosylate Ph C C Na| + L 3 H3O| (c) CH3C' C MgBr ethylene oxide (d) L '+ Br(CH2)5Br HC C_ Na|(excess) + 3 14.19 Explain why graduate student Choke Fumely, in attempting to synthesize 4,4-dimethyl-2- pentyne using the reaction of H3C C'C_ Na| with tert-butyl bromide, obtained none of the desired product. L 3 14.20 Propose a synthesis of 4,4-dimethyl-2-pentyne (the compound in Problem 14.19) from an alkyl halide and an alkyne. 14.21 Outline two different preparations of 2-pentyne that involve an alkyne and an alkyl halide. 14.22 Propose another pair of reactants that could be used to prepare 2-heptyne (the product in Eq. 14.28). 14.8 ORGANIC SYNTHESIS USING ALKYNES Let’s tie together what we’ve learned about alkyne reactions and organic synthesis. The solu- tion to Study Problem 14.2 requires all of the fundamental operations of organic synthesis: the formation of carbon–carbon bonds, the transformation of functional groups, and the establish- ment of stereochemistry (Sec. -

“Alkenyl Nonaflates from Carbonyl Compounds: New Synthesis, Elimination Reactions, and Systematic Study of Heck and Sonogashira Cross-Couplings”
“Alkenyl nonaflates from carbonyl compounds: New synthesis, elimination reactions, and systematic study of Heck and Sonogashira cross-couplings” A thesis submitted to the Freie Universität Berlin for the degree of Dr. rer. nat. Faculty of Chemistry and Biochemistry 2009 Michael Alexander Kolja Vogel Department of Biology, Chemistry and Pharmacy FU Berlin 1. Gutachter: Prof. Dr. C. B. W. Stark 2. Gutachter: Prof. Dr. H.-U. Reißig Promotionsdatum: 19.06.2009 Contents Contents Contents 3 Abbreviations 7 Declaration and Copyright Statement 9 The Author 12 Acknowledgements 13 Abstract / Zusammenfassung 14 Introduction and Objective 16 Chapter 1 Alkenyl nonaflates from enolizable carbonyl precursors – 25 methodology, preparation, and elimination reactions 1.1. Purification of NfF and compatibility experiments with bases 26 1.2. Application of the internal quenching protocol for the 30 preparation of cyclic alkenyl nonaflates 1.3. Reactions of acyclic ketones with NfF and phosphazene bases 36 1.3.1 General remarks 36 1.3.2. Synthesis of alkynes: reactivity and selectivity 38 1.4. The formation of allenes 45 1.5. Conversion of aldehydes with NfF and phosphazene bases 50 1.5.1. Alkenyl nonaflate formation 50 1.5.2. Formation of terminal alkynes 52 1.6. Conclusions 54 Chapter 2 The alkenyl nonaflates in the Heck reaction – 56 methodology and reactivity 3 Contents 2.1. General remarks 57 2.2. Methodology and initial experiments 59 2.3. Systematic investigations 61 2.3.1. The solvent effect 61 2.3.2. The effect of different bases 65 2.3.3. The effect of additives 66 2.3.4. The effect of triphenylphosphine 71 2.3.5. -
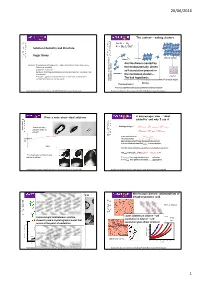
Roger Davey + Critical Nucleus Dimers Are the Dimers Created by Content: Studying and Defining Solute- Solute Interactions in the Liquid Phase
20/06/2016 The context – making clusters A + A A 2 K = [A ] / [A] 2 Solution Chemistry and Structure 2 Roger Davey + Critical nucleus dimers Are the dimers created by Content: Studying and defining solute- solute interactions in the liquid phase. Techniques available. thermodynamically driven Speciation in solution. self association present in Do these (thermodynamic features have) an impact on nucleation rate tetramers or outcome? monomers the nucleation clusters – Examples – glycine/saccharin/benzoic acid/tetrolic acid/mandelic crystal acid/paminobenzoic acid/co-crystals. The link hypothesis . Equilibria in supersaturated homogeneous solution Formation and Growth of critical nuclei Kinetics Thermodynamics Are these equilibria and quasi equilibria in anyway related? Nucleation Summer School June 20-24 th 2016 University of Strathclyde Nucleation Summer School June 20-24 th 2016 University of Strathclyde First a note about ideal solutions A macroscopic view - ‘ideal solubility’ and why I use it. Enthalpy changes: The usual way: ∆Hsolution = ∆H sublimation + ∆H solvation dissolve solid in ∆H = ∆H + ∆H solvent solution fusion mixing In an ideal solution ∆H mixing = 0. compositi This means that (Es-s + Esolv-solv )/2 = Es-solv on Real solutions don’t have this exact balance and in a non-ideal solution ∆H mixing is thus non zero. time And the ideal solubility is given by a very familiar equation: ln(xideal )={ΔHF(1/T m-1/T)+ ΔCp[T m/T – ln(T m/T)-1]}/R The ideal way: melt solid and mix with solvent. If x > xideal then negative deviation …….solvation If x < xideal then positive deviation………aggregation. Nucleation Summer School June 20-24 th 2016 University of Strathclyde Nucleation Summer School June 20-24 th 2016 University of Strathclyde Macroscopic picture: polymorphism of Macroscopic picture: saccharin dihydroxybenzoic acid. -
Chemical Specificity in Short-Chain Fatty Acids and Their Analogues in Increasing Osmotic Fragility in Rat Erythrocytes in Vitro
Chemical specificity in short-chain fatty acids and their analogues in increasing osmotic fragility in rat erythrocytes in Title vitro. Author(s) Mineo, Hitoshi; Hara, Hiroshi Biochimica et Biophysica Acta (BBA) - Biomembranes, 1768(6), 1448-1453 Citation https://doi.org/10.1016/j.bbamem.2007.02.008 Issue Date 2007-06 Doc URL http://hdl.handle.net/2115/28208 Type article (author version) File Information BBA1768-6.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 1 Chemical specificity in short-chain fatty acids and their analogues in increasing osmotic 2 fragility in rat erythrocytes in vitro 3 4 Hitoshi Mineo, Hiroshi Hara* 5 6 Division of Applied Bioscience, Graduate School of Agriculture, Hokkaido University, 7 Hokkaido 060-8589, Japan 8 9 10 *Corresponding author. 11 Hiroshi HARA Ph.D. 12 Division of Applied Bioscience, 13 Graduate School of Agriculture, 14 Hokkaido University, 15 Kita-9, Nishi-9, Sapporo, 16 Hokkaido 060-8589, 17 Tel.: +81-11-706-3352; 18 fax: +81-11-706-2504. 19 E-mail address: [email protected] 20 21 22 23 24 25 2 1 Abstract 2 3 We examined the role of the chemical specificity of short-chain fatty acids 4 (SCFAs) and their derivatives in increasing osmotic fragility (OF) in rat red blood cells 5 (RBCs). Except for formic acid, normal SCFAs with 2 to 8 carbons increased the OF in 6 rat RBCs with increasing number of hydrocarbons in a dose-dependent manner. 7 Replacement of the carboxylic group with sulfonic group inhibited, but did not abolish, 8 the SCFA-mediated increase in OF. -

S1 Supporting Information Copper-Catalyzed
Supporting Information Copper-Catalyzed Semihydrogenation of Internal Alkynes with Molecular Hydrogen Takamichi Wakamatsu, Kazunori Nagao, Hirohisa Ohmiya*, and Masaya Sawamura* Department of Chemistry, Faculty of Science, Hokkaido University, Sapporo 060-0810, Japan Table of Contents Instrumentation and Chemicals S1 Characterization Data for Alkynes S1–S2 Procedure for the Copper-Catalyzed Semihydrogenation of Alkynes S2 Characterization Data for Alkenes S3–S5 References S5 NMR Spectra S6–S31 Instrumentation and Chemicals NMR spectra were recorded on a JEOL ECX-400, operating at 400 MHz for 1H NMR and 100.5 13 1 13 MHz for C NMR. Chemical shift values for H and C are referenced to Me4Si and the residual solvent resonances, respectively. Mass spectra were obtained with Thermo Fisher Scientific Exactive, JEOL JMS-T100LP or JEOL JMS-700TZ at the Instrumental Analysis Division, Equipment Management Center, Creative Research Institution, Hokkaido University. TLC analyses were performed on commercial glass plates bearing 0.25-mm layer of Merck Silica gel 60F254. Silica gel (Kanto Chemical Co., Silica gel 60 N, spherical, neutral) was used for column chromatography. Materials were obtained from commercial suppliers or prepared according to standard procedure unless otherwise noted. CuCl was purchased from Aldrich Chemical Co., stored under nitrogen, and used as it is. NatOBu, octane and 6-dodecyne 1a were purchased from TCI Chemical Co., stored under nitrogen, and used as it is. Diphenylacetylene 1j was purchased from Wako Chemical Co., stored under nitrogen, and used as it is. 1,4-Dioxane was purchased from Kanto Chemical Co., distilled from sodium/benzophenone and stored over 4Å molecular sieves under nitrogen. -

Chapter 8 - Alkynes: an Introduction to Organic Synthesis
Chapter 8 - Alkynes: An Introduction to Organic Synthesis Draw structures corresponding to each of the following names. 1. ethynylcyclopropane Answer: CCH 2. 3,10-dimethyl-6-sec-butylcyclodecyne Answer: 3. 4-bromo-3,3-dimethyl-1-hexen-5-yne CH3 Br Answer: H 2C CH C CH C C H CH3 4. acetylene Answer: H CCH Provide names for each compound below. CH3 5. CH3C CCHCH2CH2CH3 Answer: 4-methyl-2-heptyne CH 3 6. CCH Answer: 1-ethynyl-2-methylcyclopentane Test Items for McMurry’s Organic Chemistry, Seventh Edition 59 The compound below has been isolated from the safflower plant. Consider its structure to answer the following questions. H H CCCCCCCC H H3C C C C H H C H H 7. What is the molecular formula for this natural product? Answer: C13H10 8. What is the degree of unsaturation for this compound? Answer: We can arrive at the degree of unsaturation for a structure in two ways. Since we know that the degree of unsaturation is the number of rings and/or multiple bonds in a compound, we can simply count them. There are three double bonds (3 degrees) and three triple bonds (six degrees), so the degree of unsaturation is 9. We can verify this by using the molecular formula, C13H10, to calculate a degree of unsaturation. The saturated 13-carbon compound should have the base formula C13H28, so (28 - 10) ÷ 2 = 18 ÷ 2 = 9. 9. Assign E or Z configuration to each of the double bonds in the compound. Answer: H H E CCCCCCCCE H H3C C C C H H C H H 10. -

The Chemistry of Alkynes
14_BRCLoudon_pgs4-2.qxd 11/26/08 9:04 AM Page 644 14 14 The Chemistry of Alkynes An alkyne is a hydrocarbon containing a carbon–carbon triple bond; the simplest member of this family is acetylene, H C'C H. The chemistry of the carbon–carbon triple bond is similar in many respects toL that ofL the carbon–carbon double bond; indeed, alkynes and alkenes undergo many of the same addition reactions. Alkynes also have some unique chem- istry, most of it associated with the bond between hydrogen and the triply bonded carbon, the 'C H bond. L 14.1 NOMENCLATURE OF ALKYNES In common nomenclature, simple alkynes are named as derivatives of the parent compound acetylene: H3CCC' H L L methylacetylene H3CCC' CH3 dimethylacetyleneL L CH3CH2 CC' CH3 ethylmethylacetyleneL L Certain compounds are named as derivatives of the propargyl group, HC'C CH2 , in the common system. The propargyl group is the triple-bond analog of the allyl group.L L HC' C CH2 Cl H2CA CH CH2 Cl L L LL propargyl chloride allyl chloride 644 14_BRCLoudon_pgs4-2.qxd 11/26/08 9:04 AM Page 645 14.1 NOMENCLATURE OF ALKYNES 645 We might expect the substitutive nomenclature of alkynes to be much like that of alkenes, and it is. The suffix ane in the name of the corresponding alkane is replaced by the suffix yne, and the triple bond is given the lowest possible number. H3CCC' H CH3CH2CH2CH2 CC' CH3 H3C CH2 C ' CH L L L L L L L propyne 2-heptyne 1-butyne H3C CH C ' C CH3 HC' C CH2 CH2 C' C CH3 L L L L 1,5-heptadiyneLL L "CH3 4-methyl-2-pentyne Substituent groups that contain a triple bond (called alkynyl groups) are named by replac- ing the final e in the name of the corresponding alkyne with the suffix yl. -

The Synthesis of a Polydiacetylene to Create a Novel Sensory Material
SELDE, KRISTEN A., M.S. The Synthesis of a Polydiacetylene to Create a Novel Sensory Material. (2007) Directed by Dr. Darrell Spells. 47pp. Sensory materials that respond to chemical and mechanical stimuli are under development in many laboratories. There are many significant uses of polydiacetylene compounds as sensory material. They have been applied to drug delivery, drug design, biomolecule development, cosmetics, and national security. In this study, experiments were carried out toward the development of a novel sensory material based on the established synthetic research on polydiacetylene compounds. Synthetic routes toward sensory materials with different head groups, different carbon chains lengths, and the incorporation of molecular imprints were explored. Diacetylene moieties, which can be used for polymer vesicle formation, were prepared by two main routes. In one route, 1-iodo-1-octyne and 1-iodo-1-dodecyne were prepared as starting materials for the synthesis of two diacetylene compounds (Diacetylene I and Diacetylene II). In the other route, a mesityl alkyne was used to prepare 5-iodo-1-pentyne, which was then used to prepare a triethylamino alkyne. This in turn was used to synthesize a diacetylene (Diacetylene III). Although each diacetylene product was formed, purification by column chromatography was found to be difficult. Experiments in vesicle formation, with and without molecular imprints, were also carried out using commercially available diacetylenes . THE SYNTHESIS OF A POLYDIACETYLENE TO CREATE A NOVEL SENSORY -

The Molecular Level Modification of Surfaces: from Self-Assembled Monolayers to Complex Molecular Assemblies
University of Wollongong Research Online Australian Institute for Innovative Materials - Papers Australian Institute for Innovative Materials 1-1-2011 The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies J Justin Gooding University of New South Wales, [email protected] Simone Ciampi University of New South Wales, [email protected] Follow this and additional works at: https://ro.uow.edu.au/aiimpapers Part of the Engineering Commons, and the Physical Sciences and Mathematics Commons Recommended Citation Gooding, J Justin and Ciampi, Simone, "The molecular level modification of surfaces: From self- assembled monolayers to complex molecular assemblies" (2011). Australian Institute for Innovative Materials - Papers. 1853. https://ro.uow.edu.au/aiimpapers/1853 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies Abstract The modification of surfaces with self-assembled monolayers (SAMs) containing multiple different molecules, or containing molecules with multiple different functional components, or both, has become increasingly popular over the last two decades. This explosion of interest is primarily related to the ability to control the modification of interfaces with something approaching molecular level control and to the ability to characterise the molecular constructs by which the surface is modified. Over this time the level of sophistication of molecular constructs, and the level of knowledge related to how to fabricate molecular constructs on surfaces have advanced enormously. This critical review aims to guide researchers interested in modifying surfaces with a high degree of control to the use of organic layers. -

United States Patent (19) 11 4,027,037 Siegle Et Al
United States Patent (19) 11 4,027,037 Siegle et al. (45) May 31, 1977 (54) N-SUBSTITUTED B-AMINOCROTONIC 57) ABSTRACT ACID ESTERS N-substituted (3-aminocrotonic acid esters of the for mula (75) Inventors: Peter Siegle, Cologne; Klaus Sasse, Schildgen; Peter Rössler, Cologne, all of Germany CH. COOR: (I) RC=CCN H (73) Assignee: Bayer Aktiengesellschaft, R11 Leverkusen, Germany in which (22 Filed: Mar. 12, 1975 R is hydrogen, or alkyl or alkenyl with up to 4 car bon atoms, (21) Appl. No.: 557,698 R’ is straight-chain or branched alkyl or alkenyl with up to 9 carbon atoms, optionally interrupted by O 30 Foreign Application Priority Data or S, and optionally substituted by halogen, phe Mar. 26, 1974 Germany .......................... 2414456 noxy, dimethylamino, cyclic alkyl or alkenyl with up to 8 carbon atoms which are optionally substi (52) U.S. C. ......................... 424/314; 260/239 BE; tuted by halogen, dimethylamino, or alkyl, alkoxy 260/239 BF; 260/247.2 B; 260/268 R; or alkylthio with up to 4 carbon atoms, phenyl 260/293.88; 260/326.43; 260/340.5; which is optionally substituted by halogen, dimeth 260/347.4; 260/468 G; 260/468 J; 260/470; ylamino, phenoxy, or alkyl, alkenyl, acyl, alkoxy, 260/471 A; 260/479 S; 260/481 R; 260/482 alkenoxy or alkynoxy containing up to 4 carbon R; 424/244; 424/2 48.52; 424/250; 424/267; atoms, or a 5- or 6-membered heterocyclic ring 424/274; 424/282; 424/285; 424/299; containing at least one O, S or N hetero-atom and 424/309; 424/248.55 optionally substituted by halogen, alkyl or alkoxy containing up to 4 carbon atoms or R is further (51 int. -

Strategies for the Synthesis of Ynamides
I. SYNTHESIS OF INDOLINES AND INDOLES VIA INTRAMOLECULAR [4 + 2] CYCLOADDITION OF YNAMIDES AND CONJUGATED ENYNES II. SYNTHESIS OF NITROGEN HETEROCYCLES IN SUPERCRITICAL CARBON DIOXIDE by Joshua Ross Dunetz B. A., Chemistry Haverford College, 2000 SUBMITTED TO THE DEPARTMENT OF CHEMISTRY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY September 2005 © Massachusetts Institute of Technology, 2005 All rights reserved Signature of Author .................... ·Department of Chemistry September 1, 2005 a( / Certified by ................................... ................................ Rick L. Danheiser Arthur C. Cope Professor of Chemistry, Thesis Supervisor Acceptedby......................... ............................................ I.... 7 Robert W. Field MASSACHUSETSINSTn '.vE I F TrwfhNl erv-v I Chairman, Department Committee on Graduate Students OCT 1 2005 d }cl/CF , LIBRARIES ~ This doctoral thesis has been examined by a committee in the Department of Chemistry as follows: Professor Timothy F. Jamison . ... Chairman Professor Rick L. Danheiser ........... ... ............................ Thesis Supervisor Professor Barbara Imperiali. ...... ................................................ 2 ACKNOWLEDGMENTS All acknowledgments must begin with my thesis advisor, Rick Danheiser. I first remember meeting him at the Cambridge Brewing Company during recruiting weekend five years ago, and we sat for hours in the restaurant discussing the merits of the 2000 New York Mets and whether one of our favorite baseball teams had a chance to make the playoffs that year. Ultimately, I decided to attend MIT with the hope of joining his group, and during my time in his laboratory Rick has been an excellent mentor and chemistry role model. I continue to be amazed not only by the extent of his knowledge, but also by his ability to articulate chemical principles in an easy and straightforward manner.