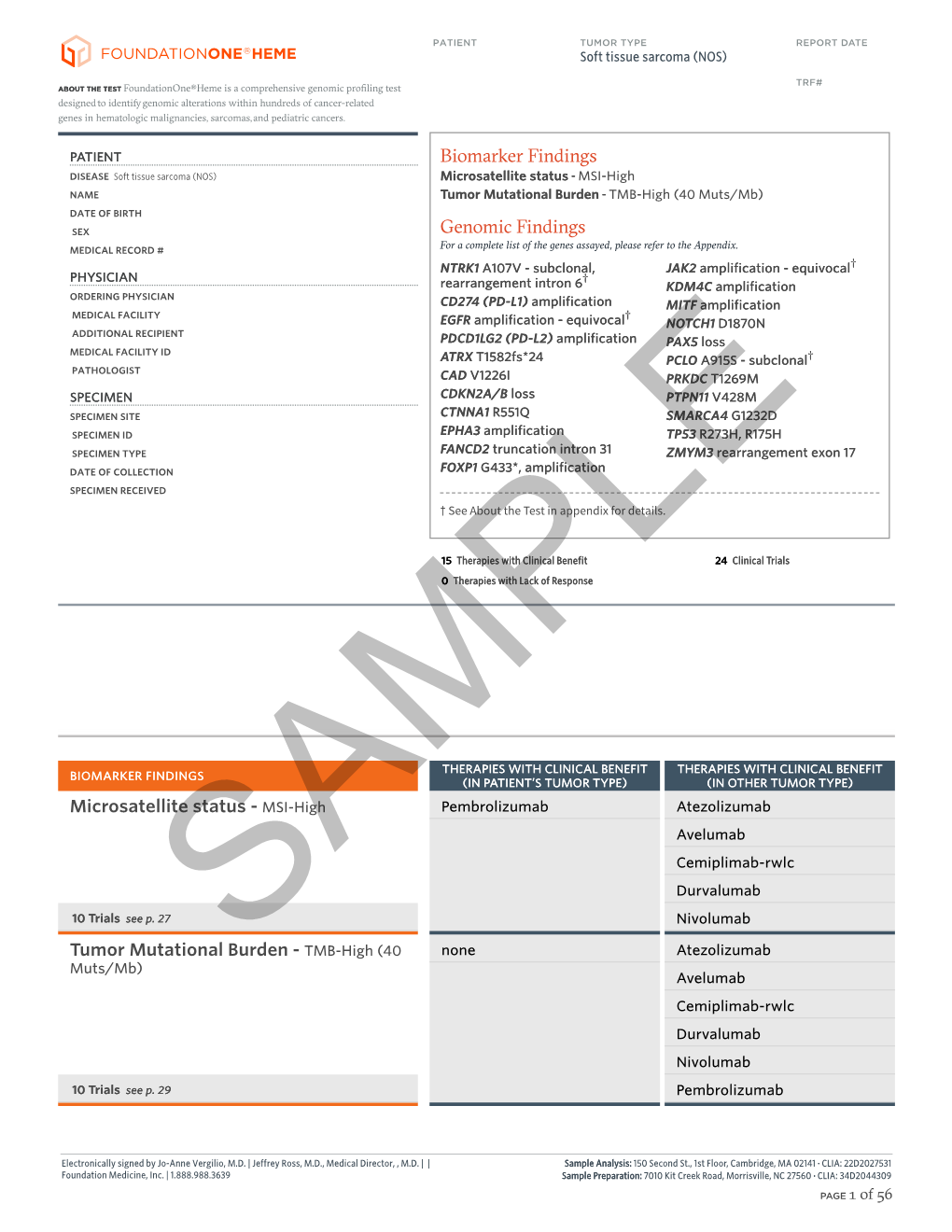
F1H Soft Tissue Sarcoma (NOS) Sample Report.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FLT3 Inhibitors in Acute Myeloid Leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2*
Wu et al. Journal of Hematology & Oncology (2018) 11:133 https://doi.org/10.1186/s13045-018-0675-4 REVIEW Open Access FLT3 inhibitors in acute myeloid leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2* Abstract FLT3 mutations are one of the most common findings in acute myeloid leukemia (AML). FLT3 inhibitors have been in active clinical development. Midostaurin as the first-in-class FLT3 inhibitor has been approved for treatment of patients with FLT3-mutated AML. In this review, we summarized the preclinical and clinical studies on new FLT3 inhibitors, including sorafenib, lestaurtinib, sunitinib, tandutinib, quizartinib, midostaurin, gilteritinib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG 925, TTT-3002, and FF-10101. New generation FLT3 inhibitors and combination therapies may overcome resistance to first-generation agents. Keywords: FMS-like tyrosine kinase 3 inhibitors, Acute myeloid leukemia, Midostaurin, FLT3 Introduction RAS, MEK, and PI3K/AKT pathways [10], and ultim- Acute myeloid leukemia (AML) remains a highly resist- ately causes suppression of apoptosis and differentiation ant disease to conventional chemotherapy, with a me- of leukemic cells, including dysregulation of leukemic dian survival of only 4 months for relapsed and/or cell proliferation [11]. refractory disease [1]. Molecular profiling by PCR and Multiple FLT3 inhibitors are in clinical trials for treat- next-generation sequencing has revealed a variety of re- ing patients with FLT3/ITD-mutated AML. In this re- current gene mutations [2–4]. New agents are rapidly view, we summarized the preclinical and clinical studies emerging as targeted therapy for high-risk AML [5, 6]. on new FLT3 inhibitors, including sorafenib, lestaurtinib, In 1996, FMS-like tyrosine kinase 3/internal tandem du- sunitinib, tandutinib, quizartinib, midostaurin, gilteriti- plication (FLT3/ITD) was first recognized as a frequently nib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG mutated gene in AML [7]. -

IGF System in Sarcomas: a Crucial Pathway with Many Unknowns to Exploit for Therapy
61 1 Journal of Molecular C Mancarella and Targeting IGF system in sarcoma 61:1 T45–T60 Endocrinology K Scotlandi THEMATIC REVIEW 40 YEARS OF IGF1 IGF system in sarcomas: a crucial pathway with many unknowns to exploit for therapy Caterina Mancarella and Katia Scotlandi Experimental Oncology Lab, CRS Development of Biomolecular Therapies, Orthopaedic Rizzoli Institute, Bologna, Italy Correspondence should be addressed to K Scotlandi: [email protected] This paper forms part of a special section on 40 Years of IGF1. The guest editors for this section were Derek LeRoith and Emily Gallagher. Abstract The insulin-like growth factor (IGF) system has gained substantial interest due to its Key Words involvement in regulating cell proliferation, differentiation and survival during anoikis f sarcomas and after conventional and targeted therapies. However, results from clinical trials have f IGF system been largely disappointing, with only a few but notable exceptions, such as trials targeting f targeted therapy sarcomas, especially Ewing sarcoma. This review highlights key studies focusing on IGF f clinical trials signaling in sarcomas, specifically studies underscoring the properties that make this system an attractive therapeutic target and identifies new relationships that may be exploited. This review discusses the potential roles of IGF2 mRNA-binding proteins (IGF2BPs), discoidin domain receptors (DDRs) and metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) in regulating the IGF system. Deeper investigation of these novel regulators of the IGF system may help us to further elucidate the spatial and temporal control of the IGF Journal of Molecular axis, as understanding the control of this axis is essential for future clinical studies. -

Therapeutic Targeting of the IGF Axis
cells Review Therapeutic Targeting of the IGF Axis Eliot Osher and Valentine M. Macaulay * Department of Oncology, University of Oxford, Oxford, OX3 7DQ, UK * Correspondence: [email protected]; Tel.: +44-1865617337 Received: 8 July 2019; Accepted: 9 August 2019; Published: 14 August 2019 Abstract: The insulin like growth factor (IGF) axis plays a fundamental role in normal growth and development, and when deregulated makes an important contribution to disease. Here, we review the functions mediated by ligand-induced IGF axis activation, and discuss the evidence for the involvement of IGF signaling in the pathogenesis of cancer, endocrine disorders including acromegaly, diabetes and thyroid eye disease, skin diseases such as acne and psoriasis, and the frailty that accompanies aging. We discuss the use of IGF axis inhibitors, focusing on the different approaches that have been taken to develop effective and tolerable ways to block this important signaling pathway. We outline the advantages and disadvantages of each approach, and discuss progress in evaluating these agents, including factors that contributed to the failure of many of these novel therapeutics in early phase cancer trials. Finally, we summarize grounds for cautious optimism for ongoing and future studies of IGF blockade in cancer and non-malignant disorders including thyroid eye disease and aging. Keywords: IGF; type 1 IGF receptor; IGF-1R; cancer; acromegaly; ophthalmopathy; IGF inhibitor 1. Introduction Insulin like growth factors (IGFs) are small (~7.5 kDa) ligands that play a critical role in many biological processes including proliferation and protection from apoptosis and normal somatic growth and development [1]. IGFs are members of a ligand family that includes insulin, a dipeptide comprised of A and B chains linked via two disulfide bonds, with a third disulfide linkage within the A chain. -

Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy
cancers Review Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy Laura Boyero 1 , Amparo Sánchez-Gastaldo 2, Miriam Alonso 2, 1 1,2,3, , 1,2, , José Francisco Noguera-Uclés , Sonia Molina-Pinelo * y and Reyes Bernabé-Caro * y 1 Institute of Biomedicine of Seville (IBiS) (HUVR, CSIC, Universidad de Sevilla), 41013 Seville, Spain; [email protected] (L.B.); [email protected] (J.F.N.-U.) 2 Medical Oncology Department, Hospital Universitario Virgen del Rocio, 41013 Seville, Spain; [email protected] (A.S.-G.); [email protected] (M.A.) 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), 28029 Madrid, Spain * Correspondence: [email protected] (S.M.-P.); [email protected] (R.B.-C.) These authors contributed equally to this work. y Received: 16 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. This has led to the development of several therapeutic strategies focused in this direction. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease. Abstract: After several decades without maintained responses or long-term survival of patients with lung cancer, novel therapies have emerged as a hopeful milestone in this research field. -

Cytokine Signaling in Tumor Progression
Immune Netw. 2017 Aug;17(4):214-227 https://doi.org/10.4110/in.2017.17.4.214 pISSN 1598-2629·eISSN 2092-6685 Review Article Cytokine Signaling in Tumor Progression Myungmi Lee, Inmoo Rhee* Department of Bioscience and Biotechnology, Sejong University, Seoul 05006, Korea Received: Apr 13, 2017 ABSTRACT Revised: Jun 22, 2017 Accepted: Jun 25, 2017 Cytokines are molecules that play critical roles in the regulation of a wide range of normal *Correspondence to functions leading to cellular proliferation, differentiation and survival, as well as in Inmoo Rhee specialized cellular functions enabling host resistance to pathogens. Cytokines released Department of Bioscience and Biotechnology, in response to infection, inflammation or immunity can also inhibit cancer development Sejong University, 209 Neungdong-ro, and progression. The predominant intracellular signaling pathway triggered by cytokines Gwangjin-gu, Seoul 05006, Korea. is the JAK-signal transducer and activator of transcription (STAT) pathway. Knockout mice Tel: +82-2-6935-2432 E-mail: [email protected] and clinical human studies have provided evidence that JAK-STAT proteins regulate the immune system, and maintain immune tolerance and tumor surveillance. Moreover, aberrant Copyright © 2017. The Korean Association of activation of the JAK-STAT pathways plays an undeniable pathogenic role in several types Immunologists of human cancers. Thus, in combination, these observations indicate that the JAK-STAT This is an Open Access article distributed under the terms of the Creative Commons proteins are promising targets for cancer therapy in humans. The data supporting this view Attribution Non-Commercial License (https:// are reviewed herein. creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial Keywords: Cytokine; JAK-STAT; Cancer; Kinase inhibitor use, distribution, and reproduction in any medium, provided the original work is properly cited. -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Lestaurtinib Enhances the Antitumor Efficacy of Chemotherapy in Murine Xenograft Models of Neuroblastoma
Published OnlineFirst February 23, 2010; DOI: 10.1158/1078-0432.CCR-09-1531 Published Online First on February 23, 2010 as 10.1158/1078-0432.CCR-09-1531 Cancer Therapy: Preclinical Clinical Cancer Research Lestaurtinib Enhances the Antitumor Efficacy of Chemotherapy in Murine Xenograft Models of Neuroblastoma Radhika Iyer1, Audrey E. Evans1,3, Xiaoxue Qi1, Ruth Ho1, Jane E. Minturn1,3, Huaqing Zhao2, Naomi Balamuth1, John M. Maris1,3, and Garrett M. Brodeur1,3 Abstract Purpose: Neuroblastoma, a common pediatric tumor of the sympathetic nervous system, is character- ized by clinical heterogeneity. The Trk family neurotrophin receptors play an important role in this behavior. Expression of TrkA is associated with favorable clinical features and outcome, whereas TrkB expression is associated with an unfavorable prognosis. We wanted to determine if the Trk-selective inhibitor lestaurtinib had therapeutic efficacy in a preclinical neuroblastoma model. Experimental Design: We performed intervention trials of lestaurtinib alone or in combination with other agents in TrkB-overexpressing neuroblastoma xenograft models. Results: Lestaurtinib alone significantly inhibited tumor growth compared to vehicle-treated animals [P = 0.0004 for tumor size and P = 0.011 for event-free survival (EFS)]. Lestaurtinib also enhanced the antitumor efficacy of the combinations of topotecan plus cyclophosphamide (P < 0.0001 for size and P < 0.0001 for EFS) or irinotecan plus temozolomide (P = 0.011 for size and P = 0.012 for EFS). There was no additive benefit of combining either 13-cis-retinoic acid or fenretinide with lestaurtinib compared to lestaurtinib alone. There was dramatic growth inhibition combining lestaurtinib with bevacizumab (P < 0.0001), but this combination had substantial systemic toxicity. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell. -

Agents Available Under CTEP Collaborative Agreements for Clinical and Non-Clinical Studies 1 As of 7/28/2021
Agents Available Under CTEP Collaborative Agreements for Clinical and Non-clinical Studies 1 as of 7/28/2021 Pharmaceutical Agent Name Alternate Name Collaborator NSC Number Drug Monitor Mechanism of Action Targets Classes abemaciclib LY2835219 Eli Lilly 783671 Piekarz CDK4/6 inhibitor CDK4/6 Small Molecule AMG510 Amgen 825510 Wright Inhibits G12C-mutated KRAS mutated KRAS protein Small Molecule Anti cell surface glycoprotein mesothelin conjugated to anetumab maytansinoid DM4 with potential antineoplastic Antibody-Drug Conjugate; ravtansine* BAY 94-9343 Bayer 791065 Moscow activity mesothelin Monoclonal Antibody anti-apoptotic Bcl-2 family Inhibits B-cell lymphoma 2 (Bcl-2) and B-cell proteins, including Bcl-2, Bcl-xL, APG-1252*** Pelcitoclax Ascentage 831685 Gore lymphoma – extra-large (Bcl-xL) Bcl-w, and Mcl-1 Small Molecule Combination of cedazuridine and ASTX727 decitabine Astex Pharmaceuticals 820631 Piekarz DNA methyltransferase (DNMT) inhibitor DNA methyltransferase Small Molecule Targets PD-L1 expressed on tumor and infiltrating programmed cell death ligand 1 Atezolizumab MPDL3280A Genentech 783608 Sharon immune cells, preventing binding to PD-1 and B7.1 (PD-L1) Monoclonal Antibody AZD5363 Capivasertib AstraZeneca 782347 Sandlund Inhibits all AKT isoforms AKT Small Molecule Inhibits Ataxia Telangiectasia and Rad3 related (ATR) AZD6738 AstraZeneca 802785 Gore serine/threonine protein kinase ATR Small Molecule Inhibitor of ataxia telangiectasia mutated and Rad3- BAY1895344 Bayer 810486 Gore related (ATR) kinase ATR Small Molecule -

AHRQ Healthcare Horizon Scanning System – Status Update Horizon
AHRQ Healthcare Horizon Scanning System – Status Update Horizon Scanning Status Update: April 2015 Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA290-2010-00006-C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 April 2015 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290-2010-00006-C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. A novel intervention may not appear in this report simply because the System has not yet detected it. The list of novel interventions in the Horizon Scanning Status Update Report will change over time as new information is collected. This should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual target technology reports are developed for those that appear to be closer to diffusion into practice in the United States. A representative from AHRQ served as a Contracting Officer’s Technical Representative and provided input during the implementation of the horizon scanning system. AHRQ did not directly participate in the horizon scanning, assessing the leads or topics, or provide opinions regarding potential impact of interventions. -

Identification of Hub Genes Associated with Prognosis, Diagnosis, Immune
Lei et al. Human Genomics (2021) 15:39 https://doi.org/10.1186/s40246-021-00341-4 PRIMARY RESEARCH Open Access Identification of hub genes associated with prognosis, diagnosis, immune infiltration and therapeutic drug in liver cancer by integrated analysis Xinyi Lei1, Miao Zhang2, Bingsheng Guan1, Qiang Chen3, Zhiyong Dong1* and Cunchuan Wang1* Abstract Background: Liver cancer is one of the most common cancers and causes of cancer death worldwide. The objective was to elucidate novel hub genes which were benefit for diagnosis, prognosis, and targeted therapy in liver cancer via integrated analysis. Methods: GSE84402, GSE101685, and GSE112791 were filtered from the Gene Expression Omnibus (GEO). Differentially expressed genes (DEGs) were identified by using the GEO2R. The GO and KEGG pathway of DEGs were analyzed in the DAVID. PPI and TF network of the DEGs were constructed by using the STRING, TRANSFAC, and Harmonizome. The relationship between hub genes and prognoses in liver cancer was analyzed in UALCAN based on The Cancer Genome Atlas (TCGA). The diagnostic value of hub genes was evaluated by ROC. The relationship between hub genes and tumor-infiltrate lymphocytes was analyzed in TIMER. The protein levels of hub genes were verified in HPA. The interaction between the hub genes and the drug were identified in DGIdb. Results: In total, 108 upregulated and 60 downregulated DEGs were enriched in 148 GO terms and 20 KEGG pathways. The mRNA levels and protein levels of CDK1, HMMR, PTTG1, and TTK were higher in liver cancer tissues compared to normal tissues, which showed excellent diagnostic and prognostic value. CDK1, HMMR, PTTG1, and TTK were positively correlated with tumor-infiltrate lymphocytes, which might involve tumor immune response.