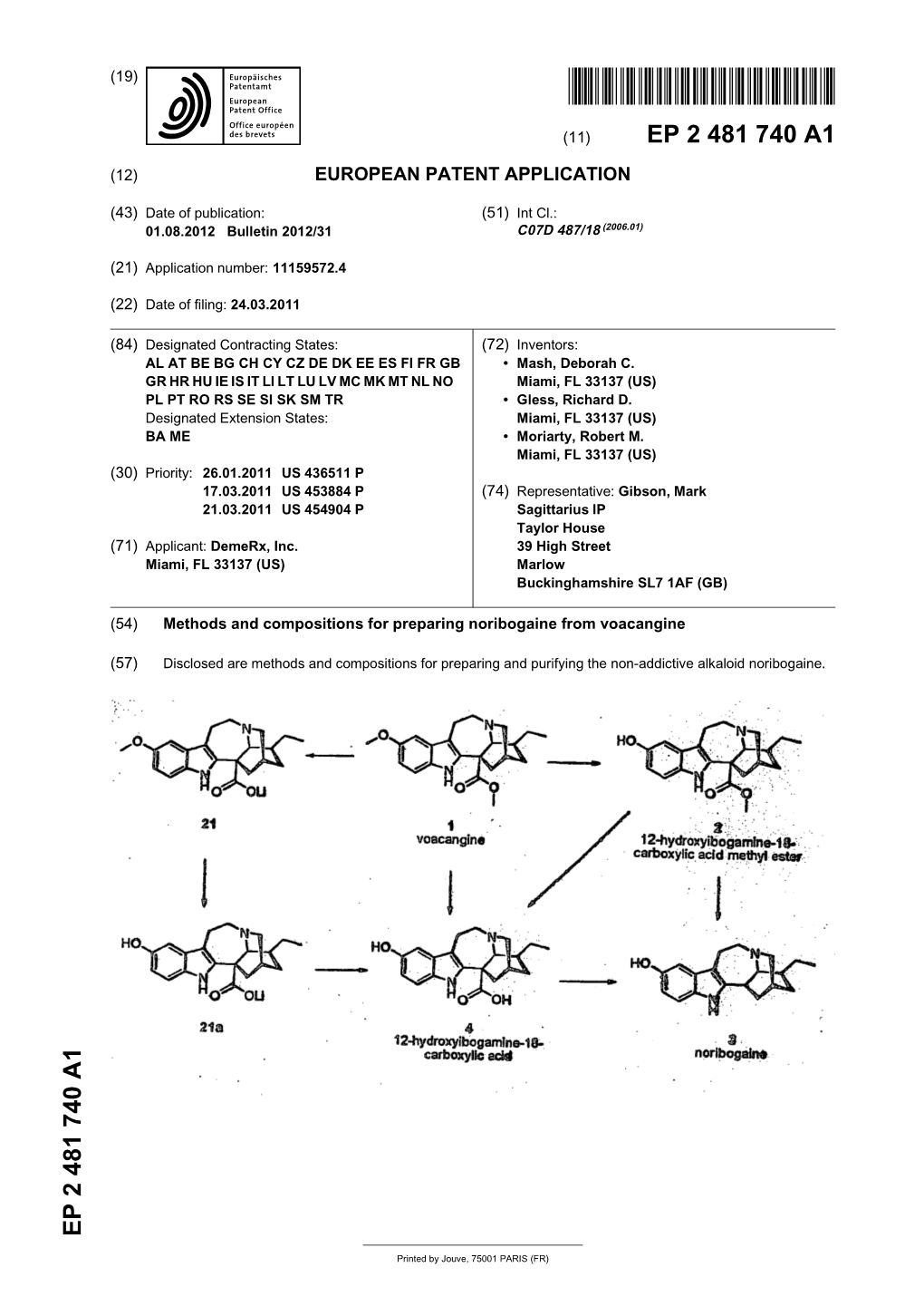
Methods and Compositions for Preparing Noribogaine from Voacangine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
(12) United States Patent (10) Patent No.: US 8,859,764 B2 Mash Et Al
US008859764B2 (12) United States Patent (10) Patent No.: US 8,859,764 B2 Mash et al. (45) Date of Patent: Oct. 14, 2014 (54) METHODS AND COMPOSITIONS FOR 4,464,378 A 8, 1984 Hussain PREPARING NORIBOGAINE FROM 1999 A 5. E. E.ea. tal VOACANGINE 4,587,243 A 5/1986 LotSof 4,604,365. A 8, 1986 O'Neill et al. (75) Inventors: Deborah C. Mash, Miami, CA (US); 4,620,977 A 1 1/1986 Strahilevitz Robert M. Moriarty, Michiana Shores, 4,626,539 A 12/1986 Aungst et al. IN (US); Richard D. Gless, Jr., 2.s sy s: A : 3. E.OCS ca.t Oakland, CA (US) 4,737,586 A 4, 1988 Potier et al. 4,806,341 A 2f1989 Chien et al. (73) Assignee: DemeRx, Inc., Ft. Lauderdale, FL (US) 4,857,523 A 8, 1989 LotSof 5,026,697 A 6, 1991 LotSof (*) Notice: Subject to any disclaimer, the term of this 5,075,341. A 12, 1991 Mendelson et al. patent is extended or adjusted under 35 Sigi A 3. 3: E. al. U.S.C. 154(b) by 0 days. 5,152.994.J. I.- A 10/1992 Lotsofranger et al. 5,283,247 A 2f1994 Dwivedi et al. (21) Appl. No.: 13/496,185 5,290,784. A 3/1994 Quet al. 5,316,759 A 5/1994 Rose et al. (22) PCT Filed: Jan. 23, 2012 5,382,657 A 1/1995 Karasiewicz et al. 5,426,112 A 6/1995 Zagon et al. (86). PCT No.: PCT/US2012/022255 5,574,0525,552,406 A 1 9,1/1996 1996 MendelsonRose et al. -

The Iboga Alkaloids
The Iboga Alkaloids Catherine Lavaud and Georges Massiot Contents 1 Introduction ................................................................................. 90 2 Biosynthesis ................................................................................. 92 3 Structural Elucidation and Reactivity ...................................................... 93 4 New Molecules .............................................................................. 97 4.1 Monomers ............................................................................. 99 4.1.1 Ibogamine and Coronaridine Derivatives .................................... 99 4.1.2 3-Alkyl- or 3-Oxo-ibogamine/-coronaridine Derivatives . 102 4.1.3 5- and/or 6-Oxo-ibogamine/-coronaridine Derivatives ...................... 104 4.1.4 Rearranged Ibogamine/Coronaridine Alkaloids .. ........................... 105 4.1.5 Catharanthine and Pseudoeburnamonine Derivatives .. .. .. ... .. ... .. .. ... .. 106 4.1.6 Miscellaneous Representatives and Another Enigma . ..................... 107 4.2 Dimers ................................................................................. 108 4.2.1 Bisindoles with an Ibogamine Moiety ....................................... 110 4.2.2 Bisindoles with a Voacangine (10-Methoxy-coronaridine) Moiety ........ 111 4.2.3 Bisindoles with an Isovoacangine (11-Methoxy-coronaridine) Moiety . 111 4.2.4 Bisindoles with an Iboga-Indolenine or Rearranged Moiety ................ 116 4.2.5 Bisindoles with a Chippiine Moiety ... ..................................... -

Leishmanicidal Activity of a Supercritical Fluid Fraction Obtained from Tabernaemontana Catharinensis ⁎ Deivid Costa Soares A, Camila G
Parasitology International 56 (2007) 135–139 www.elsevier.com/locate/parint Leishmanicidal activity of a supercritical fluid fraction obtained from Tabernaemontana catharinensis ⁎ Deivid Costa Soares a, Camila G. Pereira b, Maria Ângela A. Meireles b, Elvira Maria Saraiva a, a Departamento de Imunologia, Instituto de Microbiologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, 21941-590, Brazil b LASEFI DEA/FEA, Universidade Estadual de Campinas, Campinas, São Paulo, Cx. Postal 6121, 13001-970, Brazil Received 28 August 2006; received in revised form 11 January 2007; accepted 15 January 2007 Available online 20 January 2007 Abstract The branches and leaves of Tabernaemontana catharinensis were extracted with supercritical fluid using a mixture of CO2 plus ethanol (SFE), and the indole alkaloid enriched fraction (AF3) was selected for anti-Leishmania activity studies. We found that AF3 exhibits a potent effect against intracellular amastigotes of Leishmania amazonensis, a causative agent of New World cutaneous leishmaniasis. AF3 inhibits Leishmania survival in a dose-dependent manner, and reached 88% inhibition of amastigote growth at 100 μg/mL. The anti-parasite effect was independent of nitric oxide (NO), since AF3 was able to inhibit NO production induced by IFN-γ plus LPS. In addition, AF3 inhibited TGF-β production, which could have facilitated AF3-mediated parasite killing. The AF3 fraction obtained from SFE was nontoxic for host macrophages, as assessed by plasma membrane integrity and mitochondrial activity. We conclude that SFE is an efficient method for obtaining bioactive indole alkaloids from plant extracts. Importantly, this method preserved the alkaloid properties associated with inhibition of Leishmania growth in macrophages without toxicity to host cells. -

Roth 04 Pharmther Plant Derived Psychoactive Compounds.Pdf
Pharmacology & Therapeutics 102 (2004) 99–110 www.elsevier.com/locate/pharmthera Screening the receptorome to discover the molecular targets for plant-derived psychoactive compounds: a novel approach for CNS drug discovery Bryan L. Rotha,b,c,d,*, Estela Lopezd, Scott Beischeld, Richard B. Westkaempere, Jon M. Evansd aDepartment of Biochemistry, Case Western Reserve University Medical School, Cleveland, OH, USA bDepartment of Neurosciences, Case Western Reserve University Medical School, Cleveland, OH, USA cDepartment of Psychiatry, Case Western Reserve University Medical School, Cleveland, OH, USA dNational Institute of Mental Health Psychoactive Drug Screening Program, Case Western Reserve University Medical School, Cleveland, OH, USA eDepartment of Medicinal Chemistry, Medical College of Virginia, Virginia Commonwealth University, Richmond, VA, USA Abstract Because psychoactive plants exert profound effects on human perception, emotion, and cognition, discovering the molecular mechanisms responsible for psychoactive plant actions will likely yield insights into the molecular underpinnings of human consciousness. Additionally, it is likely that elucidation of the molecular targets responsible for psychoactive drug actions will yield validated targets for CNS drug discovery. This review article focuses on an unbiased, discovery-based approach aimed at uncovering the molecular targets responsible for psychoactive drug actions wherein the main active ingredients of psychoactive plants are screened at the ‘‘receptorome’’ (that portion of the proteome encoding receptors). An overview of the receptorome is given and various in silico, public-domain resources are described. Newly developed tools for the in silico mining of data derived from the National Institute of Mental Health Psychoactive Drug Screening Program’s (NIMH-PDSP) Ki Database (Ki DB) are described in detail. -

The Antiviral and Virucidal Activities of Voacangine and Structural Analogs Extracted from Tabernaemontana Cymosa Depend on the Dengue Virus Strain
plants Article The Antiviral and Virucidal Activities of Voacangine and Structural Analogs Extracted from Tabernaemontana cymosa Depend on the Dengue Virus Strain Laura Milena Monsalve-Escudero 1 , Vanessa Loaiza-Cano 1, Maria Isabel Zapata-Cardona 1 , Diana Carolina Quintero-Gil 1, Estiven Hernández-Mira 1, Yina Pájaro-González 2,3, Andrés Felipe Oliveros-Díaz 2, Fredyc Diaz-Castillo 2 , Wistón Quiñones 4, Sara Robledo 5 and Marlen Martinez-Gutierrez 1,* 1 Grupo de Investigación en Ciencias Animales-GRICA, Facultad de Medicina Veterinaria y Zootecnia, Universidad Cooperativa de Colombia, Bucaramanga 680005, Colombia; [email protected] (L.M.M.-E.); [email protected] (V.L.-C.); [email protected] (M.I.Z.-C.); [email protected] (D.C.Q.-G.); [email protected] (E.H.-M.) 2 Laboratorio de Investigaciones Fitoquímicas y Farmacológicas de la Universidad de Cartagena—LIFFUC, Universidad de Cartagena, Cartagena 130001, Colombia; [email protected] (Y.P.-G.); [email protected] (A.F.O.-D.); [email protected] (F.D.-C.) 3 Grupo de Investigación en Farmacia Asistencial y Farmacología, Universidad del Atlántico, Barranquilla 080001, Colombia Citation: Monsalve-Escudero, L.M.; 4 Grupo de Química Orgánica de Productos Naturales, Universidad de Antioquia, Medellín 050001, Colombia; Loaiza-Cano, V.; Zapata-Cardona, [email protected] 5 M.I.; Quintero-Gil, D.C.; Programa de Estudio y Control de Enfermedades Tropicales-PECET, Universidad de Antioquia, Medellín 050001, Colombia; [email protected] Hernández-Mira, E.; Pájaro-González, * Correspondence: [email protected]; Tel.: +57-310-5438583 Y.; Oliveros-Díaz, A.F.; Diaz-Castillo, F.; Quiñones, W.; Robledo, S.; et al. -

Bio-Guided Search of Active Indole Alkaloids from Tabernaemontana
Bioorganic Chemistry 85 (2019) 66–74 Contents lists available at ScienceDirect Bioorganic Chemistry journal homepage: www.elsevier.com/locate/bioorg Bio-guided search of active indole alkaloids from Tabernaemontana T catharinensis: Antitumour activity, toxicity in silico and molecular modelling studies Pauline Fagundes Rosalesa,b, Flavio Ferreira Marinhoa, Adriana Gowera, Marilda Chiarelloa, ⁎ Bianca Cancic, Mariana Roesch-Elyc, Favero Reisdorfer Paulad, Sidnei Mouraa, a Laboratory of Biotechnology of Natural and Synthetics Products, University of Caxias do Sul, Brazil b Federal Institute of Education, Science and Technology of Rio Grande do Sul, Campus Bento Gonçalves, Brazil c Laboratory of Genomics, Proteomics and DNA Repair, University of Caxias do Sul, Brazil d Laboratory of Research and Drugs Development, Federal University of Pampa, Brazil ARTICLE INFO ABSTRACT Keywords: Active plant metabolites have been used as prototype drugs. In this context, Tabernaemontana catharinensis Tabernaemontana catharinensis (Apocynaceae) has been highlighted because of the presence of active indole alkaloids. Thus, this study aims the A549 cell bio-guided search of T. catharinensis cytotoxic alkaloids. The chemical composition was identified by high-re- A375 cell solution mass spectrometry, and fractionation was performed by open column and preparative thin-layer Indole alkaloid chromatography, from plant stems. The enriched fractions were tested in vitro in tumour cells A375 (melanoma Toxicity cell line) and A549 (adenocarcinomic human alveolar basal epithelial cells), and non-tumour Vero cells (African green monkey kidney epithelial cells). The alkaloids identified as active were submitted to in silico toxicity prediction by ADME-Tox and OSIRIS programs and, also, to molecular docking, using topoisomerase I (PDB ID: 1SC7) by iGEMDOCK. -

Biosynthesis of an Anti-Addiction Agent from the Iboga Plant Authors: Scott C
bioRxiv preprint doi: https://doi.org/10.1101/647891; this version posted May 26, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Biosynthesis of an Anti-Addiction Agent from the Iboga Plant Authors: Scott C. Farrow1, Mohamed O. Kamileen1, Lorenzo Caputi1, Kate Bussey2, Julia E. A. Mundy2, Rory C. McAtee3, Corey R. J. Stephenson3, Sarah E. O’Connor1* Affiliations: 5 1 Max Planck Institute of Chemical Ecology, Department of Natural Product Biosynthesis, Hans- Knöll-Straße 8, 07745 Jena, Germany 2 John Innes Centre, Department of Biological Chemistry, Norwich Research Park, Norwich, NR4 7UH, UK 3 Willard Henry Dow Laboratory, Department of Chemistry, University of Michigan, 930 North 10 University Avenue, Ann Arbor, MI 48109, USA *Correspondence to: [email protected] Abstract: (–)-Ibogaine and (–)-voacangine are plant derived psychoactives that show promise as effective treatments for opioid addiction. However, these compounds are produced by hard to source plants making these chemicals difficult for broad-scale use. Here we report the complete 15 biosynthesis of (–)-voacangine, and de-esterified voacangine, which is converted to (–)-ibogaine by heating. This discovery will enable production of these compounds by synthetic biology methods. Notably, (–)-ibogaine and (–)-voacangine are of the opposite enantiomeric configuration compared to the other major alkaloids found in this natural product class. Discovery of these biosynthetic enzymes therefore demonstrates how nature generates both 20 enantiomeric series of this medically important alkaloid scaffold using closely related enzymes, including those that catalyze enantioselective formal Diels-Alder reactions. -

Visions on Iboga/Ine from the International Community
VISIONS ON IBOGA/INE FROM THE INTERNATIONAL COMMUNITY Iboga Community Engagement Initiative PHASE I REPORT December 2019 A project by: International Center for Ethnobotanical Education, Research and Service (ICEERS) Project leads Ricard Faura, PhD Andrea Langlois Advisory Committee Benjamin De Loenen, Dr. Tom Kingsley Brown, Doug Greene, Patrick Kroupa, Jeremy Weate, Hattie Wells, and Sarita Wilkins Scientific, legal and technical advice Dr. Kenneth Alper, Dr. José Carlos Bouso, Christine Fitzsimmons, Yann Guignon, Dr. Uwe Maas, Dr. Dennis McKenna, Tanea Paterson, Genís Ona, Natalia Rebollo, Dr. Constanza Sánchez, Süster Strubelt, and Clare Wilkins Editing Sarita Wilkins, Eric Swenson, Holly Weese Graphic design Àlex Verdaguer December 2019 For further information or inquiries, please email: [email protected] www.iceers.org Thank you…. As with any intitiative, this project has come to fruition thanks to the generosity of many collaborators, interviewees and survey participants. Hundreds of individuals shared knowledge, opinions, concerns, time, and visions for a future where iboga and ibogaine are valued and integrated into the global society and the rights of people connected to these practices are protected. We extend a heartfelt thank you to each and every one of them, for this report would not have been possible without their contributions. Our hope is that this report be received as the weaving of knowledge that it is, as a collection of perspectives, to be engaged with, challenged, expanded upon, and leveraged to build a brighter future. Dedication… This report is dedicated to Doug Greene. Although he is no longer with us, his gentle, persistent dedication to the healing of humanity will continue to inspire. -

The Anti-Addiction Drug Ibogaine and the Heart: a Delicate Relation
Molecules 2015, 20, 2208-2228; doi:10.3390/molecules20022208 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review The Anti-Addiction Drug Ibogaine and the Heart: A Delicate Relation Xaver Koenig * and Karlheinz Hilber * Department of Neurophysiology and Neuropharmacology, Center for Physiology and Pharmacology, Medical University of Vienna, Schwarzspanierstrasse 17, Vienna 1090, Austria * Authors to whom correspondence should be addressed; E-Mails: [email protected] (X.K.); [email protected] (K.H.); Tel.: +43-1-40160-31232 (X.K.); +43-1-40160-31230 (K.H.); Fax: +43-1-40160-931300 (X.K. & K.H.). Academic Editor: Patricia Valentao Received: 24 October 2014 / Accepted: 26 November 2014 / Published: 29 January 2015 Abstract: The plant indole alkaloid ibogaine has shown promising anti-addictive properties in animal studies. Ibogaine is also anti-addictive in humans as the drug alleviates drug craving and impedes relapse of drug use. Although not licensed as therapeutic drug and despite safety concerns, ibogaine is currently used as an anti-addiction medication in alternative medicine in dozens of clinics worldwide. In recent years, alarming reports of life-threatening complications and sudden death cases, temporally associated with the administration of ibogaine, have been accumulating. These adverse reactions were hypothesised to be associated with ibogaine’s propensity to induce cardiac arrhythmias. The aim of this review is to recapitulate the current knowledge about ibogaine’s effects on the heart and the cardiovascular system, and to assess the cardiac risks associated with the use of this drug in anti- addiction therapy. The actions of 18-methoxycoronaridine (18-MC), a less toxic ibogaine congener with anti-addictive properties, are also considered. -

Toxins and Signalling Evelyne Benoit, Françoise Goudey-Perriere, P
Toxins and Signalling Evelyne Benoit, Françoise Goudey-Perriere, P. Marchot, Denis Servent To cite this version: Evelyne Benoit, Françoise Goudey-Perriere, P. Marchot, Denis Servent. Toxins and Signalling. SFET Publications, Châtenay-Malabry, France, pp.204, 2009. hal-00738643 HAL Id: hal-00738643 https://hal.archives-ouvertes.fr/hal-00738643 Submitted on 21 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Collection Rencontres en Toxinologie © E. JOVER et al. TTooxxiinneess eett SSiiggnnaalliissaattiioonn -- TTooxxiinnss aanndd SSiiggnnaalllliinngg © B.J. LAVENTIE et al. Comité d’édition – Editorial committee : Evelyne BENOIT, Françoise GOUDEY-PERRIERE, Pascale MARCHOT, Denis SERVENT Société Française pour l'Etude des Toxines French Society of Toxinology Illustrations de couverture – Cover pictures : En haut – Top : Les effets intracellulaires multiples des toxines botuliques et de la toxine tétanique - The multiple intracellular effects of the BoNTs and TeNT. (Copyright Emmanuel JOVER, Fréderic DOUSSAU, Etienne LONCHAMP, Laetitia WIOLAND, Jean-Luc DUPONT, Jordi MOLGÓ, Michel POPOFF, Bernard POULAIN) En bas - Bottom : Structure tridimensionnelle de l’alpha-toxine staphylocoque - Tridimensional structure of staphylococcal alpha-toxin. (Copyright Benoit-Joseph LAVENTIE, Daniel KELLER, Emmanuel JOVER, Gilles PREVOST) Collection Rencontres en Toxinologie La collection « Rencontres en Toxinologie » est publiée à l’occasion des Colloques annuels « Rencontres en Toxinologie » organisés par la Société Française pour l’Etude des Toxines (SFET). -

THE Moga and VOACANGA ALKALOIDS
--CHAPTER 9-- THE mOGA AND VOACANGA ALKALOIDS W. 1. TAYLOR Research Department,OIBA Pharmaceutical Oompany, Division of OIBA Oorporation, Summit, New Jersey I. The Iboga Alkaloids. ... .... .... ..... ....... .. .. .. .. 203 A. The Structures of Ibogaine and Iboxygaine. ... ...... ...... 206 B. Ibogamine and Tabernanthine... .. .. .. .. .. .. .. .... .. .. .. .... 213 C. 18-Carbomethoxy Alkaloids _. .. 213 D. Voacryptine.. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. 217 E. Catharanthine, Cleavamine, and Velbanamine.. .. .. .. .. .. .. .. .. .. .. .. 218 F. Mass Spectra of Iboga Alkaloids.. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .... 221 G. Other Alkaloids.. .. .. .. .. .. .. .. 223 II. The Voacanga Alkaloids. ........................ 225 A. Voachalotine .... .... .. .. .. .................. ... 225 B. Vobasine, Dregamine, Tabernaemontanine, and Callichiline....... .. .. .. 228 C. Bisindoles....................................................... 229 III. Miscellaneous.. ..... .. ... .... .. .. .. .. .. 231 References _... ... .... .. .. .... .. ..... ... .... 233 I. The Ihoga Alkaloids The iboga alkaloids (Table I) presently number twelve, if their oxida- tion products are excluded, all from apocynaceous plants of the genera Oonopharyngia (Plumeria), Ervatamia, Gabunea, Stemmadenia, Taber- naemontana, Voacanga, Vinca (Lochnera, Oatharanthus), and Tabernanthe. It was from the last genus that the parent pentacyclic heterocycle, ibogamine, was first obtained. The structures of these compounds depend entirely -

Hallucinogène
Hallucinogène Un hallucinogène est une substance chimique psychotrope qui induit des hallucinations, soit, aux doses usuelles, des altérations des perceptions, de la cohérence de la pensée et de la régularité de l'humeur, mais sans confusion mentale persistante ou troubles de la mémoire[1]. Cet état est appelé état modifié de conscience par certains usagers ; il peut aussi être atteint par la spiritualité, la méditation ou à travers l'art. Les hallucinogènes forment une catégorie dans la plupart des classification des psychotropes. Il s'ensuit que leurs caractéristiques différent selon la classification utilisée. En raison de l'allure de leur impact, ils sont aussi décrits et désignés comme des perturbateurs du système nerveux central. Les effets des hallucinogènes sont clairement différents des stimulants comme la cocaïne ou les amphétamines bien qu'ils augmentent aussi la vigilance ou l'activité. La plupart des hallucinogènes appartiennent à des familles de structure chimique particulière, capables d'agir sur des emplacements spécifiques du cerveau, souvent proches des neurotransmetteurs ou des inhibiteurs, semblables à beaucoup de produits prescrits légalement. C'est ainsi qu'ils peuvent changer les qualités subjectives de la perception, de la pensée ou de l'émotion. Sommaire • 1 Histoire • 2 Chimie des hallucinogènes • 3 Pharmacologie des hallucinogènes • 4 Classification selon les effets o 4.1 Les hallucinogènes délirants o 4.2 Les hallucinogènes dissociatifs o 4.3 Les hallucinogènes psychédéliques • 5 Classification des expériences hallucinogènes • 6 Difficultés terminologiques o 6.1 Propositions de termes spécifiques • 7 Notes et références 1 – Histoire Historiquement, certaines de ces substances connaissent des utilisations rituelles ancestrales dont certaines ont survécu jusqu'à nos jours via notamment le chamanisme et certains cultes(l'Ayahuasca par exemple).