Recovery Plan for Blackburn's Sphinx Moth
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Geology of Hawaii Reefs
11 Geology of Hawaii Reefs Charles H. Fletcher, Chris Bochicchio, Chris L. Conger, Mary S. Engels, Eden J. Feirstein, Neil Frazer, Craig R. Glenn, Richard W. Grigg, Eric E. Grossman, Jodi N. Harney, Ebitari Isoun, Colin V. Murray-Wallace, John J. Rooney, Ken H. Rubin, Clark E. Sherman, and Sean Vitousek 11.1 Geologic Framework The eight main islands in the state: Hawaii, Maui, Kahoolawe , Lanai , Molokai , Oahu , Kauai , of the Hawaii Islands and Niihau , make up 99% of the land area of the Hawaii Archipelago. The remainder comprises 11.1.1 Introduction 124 small volcanic and carbonate islets offshore The Hawaii hot spot lies in the mantle under, or of the main islands, and to the northwest. Each just to the south of, the Big Island of Hawaii. Two main island is the top of one or more massive active subaerial volcanoes and one active submarine shield volcanoes (named after their long low pro- volcano reveal its productivity. Centrally located on file like a warriors shield) extending thousands of the Pacific Plate, the hot spot is the source of the meters to the seafloor below. Mauna Kea , on the Hawaii Island Archipelago and its northern arm, the island of Hawaii, stands 4,200 m above sea level Emperor Seamount Chain (Fig. 11.1). and 9,450 m from seafloor to summit, taller than This system of high volcanic islands and asso- any other mountain on Earth from base to peak. ciated reefs, banks, atolls, sandy shoals, and Mauna Loa , the “long” mountain, is the most seamounts spans over 30° of latitude across the massive single topographic feature on the planet. -

User's Guide Project: the Impact of Non-Native Predators On
User’s Guide Project: The Impact of Non-Native Predators on Pollinators and Native Plant Reproduction in a Hawaiian Dryland Ecosystem SERDP project number: RC-2432 Principal Investigators: Christina T. Liang, USDA Forest Service Clare E. Aslan, Northern Arizona University William P. Haines, University of Hawaiʻi at Mānoa Aaron B. Shiels, USDA APHIS Contributor: Manette E. Sandor, Northern Arizona University Date: 30 October 2019 Form Approved REPORT DOCUMENTATION PAGE OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing this collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Department of Defense, Washington Headquarters Services, Directorate for Information Operations and Reports (0704-0188), 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202- 4302. Respondents should be aware that notwithstanding any other provision of law, no person shall be subject to any penalty for failing to comply with a collection of information if it does not display a currently valid OMB control number. PLEASE DO NOT RETURN YOUR FORM TO THE ABOVE ADDRESS. 1. REPORT DATE (DD-MM-YYYY) 2. REPORT TYPE 3. DATES COVERED (From - To) 10-30-2019 User’s Guide 01-02-2014 to 10-30-2019 4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER User’s Guide. The Impact of Non-Native Predators on Pollinators and Native Plant Reproduction in a Hawaiian Dryland Ecosystem. -

Martha Warren Beckwith: the Kumulipo, 1951 the KUMULIPO
Hawaii: the Center of the Pacific Ethnic Studies 255 Summer 2008 Instructor: Dr. Alan E. Yabui Phone: 425-564-3083 Email: [email protected] Office Hrs: 3:30 PM, Monday thru Thursday or TBA Textbooks: Beckwith, M., (1951) Kumulipo. Honolulu: University of Hawaii Press. Also available free on the internet. See citation below. Meyer, M. A. (2003). Ho’oulu. Honolulu: “Ai Pohaku Press. Osorio, J. K. (2002). Dismembering Lahui. Honolulu: University of Hawaii Press. Free text and translation of Kumulipo on the Internet: Martha Warren Beckwith: The Kumulipo, 1951 THE KUMULIPO. A Hawaiian Creation Chant. Translated and edited with commentary by. MARTHA WARREN BECKWITH. University of Chicago Press ... www.ling.hawaii.edu/faculty/stampe/Oral-Lit/Hawaiian/Kumulipo/kumulipo-book.html - 449k - Cached - Similar pages Hawaii Geography information may be found at http://geography.about.com/library/blank/blxushi.htm . The blank outline map from this web site will be used as the Hawaii Geography Quiz map. This is a Humanities Distribution course. (May be used as either Humanities or Social Sciences if transferring to UW.) This course is designed to present Hawaii as a place where Hawaiian culture was a striving culture before the arrival of Captain James Cook at Waimea, Kauai on January 20, 1778 and the landing at Kealakekua Bay, Hawaii on January 17, 1779. After the first European contact, major changes occurred in the Hawaiian culture. When the Native Hawaiians came in physical contact with Cook’s men, the Hawaiians were introduced to human diseases which they had little or not immunity for and this started a drastic decline in the native Hawaiian population. -

Pu'u Wa'awa'a Biological Assessment
PU‘U WA‘AWA‘A BIOLOGICAL ASSESSMENT PU‘U WA‘AWA‘A, NORTH KONA, HAWAII Prepared by: Jon G. Giffin Forestry & Wildlife Manager August 2003 STATE OF HAWAII DEPARTMENT OF LAND AND NATURAL RESOURCES DIVISION OF FORESTRY AND WILDLIFE TABLE OF CONTENTS TITLE PAGE ................................................................................................................................. i TABLE OF CONTENTS ............................................................................................................. ii GENERAL SETTING...................................................................................................................1 Introduction..........................................................................................................................1 Land Use Practices...............................................................................................................1 Geology..................................................................................................................................3 Lava Flows............................................................................................................................5 Lava Tubes ...........................................................................................................................5 Cinder Cones ........................................................................................................................7 Soils .......................................................................................................................................9 -
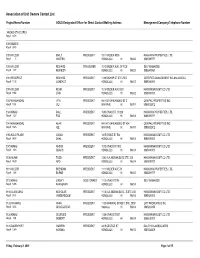
Association of Unit Owners Contact List
Association of Unit Owners Contact List Project Name/Number AOUO Designated Officer for Direct Contact/Mailing Address Management Company/Telephone Number `AKOKO AT HO`OPILI Reg.# 8073 1001 QUEEN Reg.# 7675 1001 WILDER EMILY PRESIDENT 1001 WILDER #305 HAWAIIAN PROPERTIES, LTD. Reg.# 5 WATERS HONOLULU HI 96822 8085399777 1010 WILDER RICHARD TREASURER 1010 WILDER AVE, OFFICE SELF MANAGED Reg.# 377 KENNEDY HONOLULU HI 96822 8085241961 1011 PROSPECT RICHARD PRESIDENT 1188 BISHOP ST STE 2503 CERTIFIED MANAGEMENT INC dba ASSOCI Reg.# 1130 CONRADT HONOLULU HI 96813 8088360911 1015 WILDER KEVIN PRESIDENT 1015 WILDER AVE #201 HAWAIIANA MGMT CO LTD Reg.# 1960 LIMA HONOLULU HI 96822 8085939100 1037 KAHUAMOKU VITA PRESIDENT 94-1037 KAHUAMOKU ST 3 CEN PAC PROPERTIES INC Reg.# 1551 VILI WAIPAHU HI 96797 8085932902 1040 KINAU PAUL PRESIDENT 1040 KINAU ST., #1206 HAWAIIAN PROPERTIES, LTD. Reg.# 527 FOX HONOLULU HI 96814 8085399777 1041 KAHUAMOKU ALAN PRESIDENT 94-1041 KAHUAMOKU ST 404 CEN PAC PROPERTIES INC Reg.# 1623 IGE WAIPAHU HI 96797 8085932902 1054 KALO PLACE JUANA PRESIDENT 1415 S KING ST 504 HAWAIIANA MGMT CO LTD Reg.# 5450 DAHL HONOLULU HI 96814 8085939100 1073 KINAU ANSON PRESIDENT 1073 KINAU ST 1003 HAWAIIANA MGMT CO LTD Reg.# 616 QUACH HONOLULU HI 96814 8085939100 1108 AUAHI TODD PRESIDENT 1240 ALA MOANA BLVD STE. 200 HAWAIIANA MGMT CO LTD Reg.# 7429 APO HONOLULU HI 96814 8085939100 1111 WILDER BRENDAN PRESIDENT 1111 WILDER AVE 7A HAWAIIAN PROPERTIES, LTD. Reg.# 228 BURNS HONOLULU HI 96822 8085399777 1112 KINAU LINDA Y SOLE OWNER 1112 KINAU ST PH SELF MANAGED Reg.# 1295 NAKAGAWA HONOLULU HI 96814 1118 ALA MOANA NICHOLAS PRESIDENT 1118 ALA MOANA BLVD., SUITE 200 HAWAIIANA MGMT CO LTD Reg.# 7431 VANDERBOOM HONOLULU HI 96814 8085939100 1133 WAIMANU ANNA PRESIDENT 1133 WAIMANU STREET, STE. -

Hawk Moths of North America Is Richly Illustrated with Larval Images and Contains an Abundance of Life History Information
08 caterpillars EUSA/pp244-273 3/9/05 6:37 PM Page 244 244 TULIP-TREE MOTH CECROPIA MOTH 245 Callosamia angulifera Hyalophora cecropia RECOGNITION Frosted green with shiny yellow, orange, and blue knobs over top and sides of body. RECOGNITION Much like preceding but paler or Dorsal knobs on T2, T3, and A1 somewhat globular and waxier in color with pale stripe running below set with black spinules. Paired knobs on A2–A7 more spiracles on A1–A10 and black dots on abdomen cylindrical, yellow; knob over A8 unpaired and rounded. lacking contrasting pale rings. Yellow abdominal Larva to 10cm. Caterpillars of larch-feeding Columbia tubercle over A8 short, less than twice as high as broad. Silkmoth (Hyalophora columbia) have yellow-white to Larva to 6cm. Sweetbay Silkmoth (Callosamia securifera) yellow-pink instead of bright yellow knobs over dorsum similar in appearance but a specialist on sweet bay. Its of abdomen and knobs along sides tend to be more white than blue (as in Cecropia) and are yellow abdominal tubercle over A8 is nearly three times as set in black bases (see page 246). long as wide and the red knobs over thorax are cylindrical (see page 246). OCCURRENCE Urban and suburban yards and lots, orchards, fencerows, woodlands, OCCURRENCE Woodlands and forests from Michigan, southern Ontario, and and forests from Canada south to Florida and central Texas. One generation with mature Massachusetts to northern Florida and Mississippi. One principal generation northward; caterpillars from late June through August over most of range. two broods in South with mature caterpillars from early June onward. -

Insect Pests
Crop Profile for Sweetpotatoes in Mississippi Prepared: May, 1999 General Production Information ● In 1997, sweetpotatoes were harvested from 8,400 acres in Mississippi. Production totaled 100 million lbs with a value of $18 million in 1997. Mississippi ranks fourth among states in sweetpotato production [8]. ● Commercial production is currently concentrated in Calhoun and Chickasaw Counties in north central Mississippi although sweetpotatoes are grown commercially in more than half of Mississippi’s counties. They can be grown throughout the state since all areas provide a frost-free period of more than 150 days [2]. Insect Pests Many insect pests have the potential to reduce the quality and yield of sweetpotatoes. Insects that damage the roots directly are the most trouble some and are referred to as soil insect pests. They can cause economic loss in relatively low numbers, and it is difficult to control then with insecticides because they live below the soil surface. Insects that injure the foliage reduce the yield of the plants indirectly and are referred to as foliage feeding insects. These insects can cause economic loss, but usually only at very high numbers, and it is relatively easy to control them with insecticides because they are exposed on the plant [1]. Sweetpotato Soil Insects: The sweetpotato root can be injured by several soil insects, including the sweetpotato weevil, rootworms, wireworms, white grubs, whitefringed beetles and flea beetles. The sweetpotato weevil larva is the only insect that tunnels throughout the root. Other soil insects feed on the surface on the developing sweetpotato root. The injury caused by rootworms and wireworms is similar and cannot be separated very easily. -

Further Records of Ecuadorian Sphingidae (Lepidoptera: Sphingidae) 137-141 - 1 3 7
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Neue Entomologische Nachrichten Jahr/Year: 1998 Band/Volume: 41 Autor(en)/Author(s): Racheli Luigi, Racheli Tommaso Artikel/Article: Further records of Ecuadorian Sphingidae (Lepidoptera: Sphingidae) 137-141 - 1 3 7 - Further records of Ecuadorian Sphingidae (Lepidoptera: Sphingidae) by Luigi Racheli & T ommaso Racheli Abstract Distributional records of 22 hawkmoths species of Ecuador are reported. In the past, distributional data on the ecuadorian Sphingidae were reported by Dognin (1886-1897), Campos (1931), Rothschild & J ordan (1903) and Schreiber (1978). Haxaire (1991), describing a new species of Xylophanes from Ecuador, has reported a total of 135 species collected in Ecuador. Racheli & R acheli (1994) listed 167 species, including recent collecting data for 80 species, and all the records for the country extracted from the literature, including also doubtful records such as Manduca muscosa (Rothschild & J ordan , 1903) and Xylophanes juanita Rothschild & J ordan , 1903 which are unlikely to occur in Ecuador. The total number of hawkmoths species of Ecuador may account for approximately 155-160 spe cies. Following the recent studies of Racheli & R acheli (1994, 1995), Racheli (1996) and Haxaire (1995, 1996a, 1996b), additional records of Museum specimens and of material collected in Ecuador are given herewith. All the specimens reported below are in the collection of the senior author unless otherwise stated. Abbreviations: Imbabura (IM); Esmeraldas (ES); Pichincha (PI); Ñapo (NA); Pastaza (PA); Morona Santiago (MS). EMEM = Entomologische Museum (Dr, Ulf Eitschberger ), Marktleuthen, Germany; ZSBS = Zoologische Saammlungen des Bayerischen, München, Germany. Sphinginae Manduca lefeburei lefeburei (Guérin-Ménéville , 1844) Remarks: In Ecuador, it is distributed on both sides of the Andes and according to Haxaire (1995) it coexists with Manduca andicola (Rothschild & J ordan , 1916). -

Diversidad De Esfinges (Lepidoptera: Sphingidae) En El Valle Del Río Rímac – Provincia De Lima, Huarochiri Y Cañete, Lima, Perú
SAGASTEGUIANA 6(2): 91 - 104. 2018 ISSN 2309-5644 ARTÍCULO ORIGINAL DIVERSIDAD DE ESFINGES (LEPIDOPTERA: SPHINGIDAE) EN EL VALLE DEL RÍO RÍMAC – PROVINCIA DE LIMA, HUAROCHIRI Y CAÑETE, LIMA, PERÚ DIVERSITY OF SPHINGES (LEPIDOPTERA: SPHINGIDAE) IN THE RIMAC RIVER VALLEY, LIMA, PERU Rubén A. Guzmán Pittman1 & Ricardo V. Vásquez Condori2 Asociación Científica para la Conservación de la Biodiversidad. [email protected], [email protected] RESUMEN Los lepidópteros nocturnos ostentan una gran diversidad de especies, sobresaliendo los grandes ejemplares denominados esfinges, a continuación en el presente trabajo se procede a citar y describir las especies halladas en el Valle del Rio Rímac - Departamento de Lima registrándose un total de 12 especies de la familia Sphingidae y estas dentro de dos sub familias (Macroglossini, con seis géneros) y (Sphingini con tres géneros) con un total de 9 géneros hallados, siendo estos: Hyles, Erinnyis, Pachylia, Callionima, Aellops, Eumorpha, Agrius, Cocytius y Manduca) entre las cuales la sub familia Sphingini es la más diversificada con 5 especies y 3 géneros. Palabras Clave: Entomología, Esfinges, Lepidópteros, Lima, Diversidad. ABSTRACT The nocturnal lepidoptera have a great diversity of species, with the large specimens called sphinxes standing out. In this paper, the species found in the Rímac River Valley - Department of Lima are cited and described, registering a total of 12 species of the family Sphingidae and these within two sub-families (Macroglossini, with six genera) and (Sphingini with three genera) with a total of 9 genera found, these being: Hyles, Erinnyis, Pachylia, Callionima, Aellops, Eumorpha, Agrius, Cocytius and Manduca) among which the Sphingini subfamily is the most diversified with 5 species and 3 genera. -

A Landscape-Based Assessment of Climate Change Vulnerability for All Native Hawaiian Plants
Technical Report HCSU-044 A LANDscape-bASED ASSESSMENT OF CLIMatE CHANGE VULNEraBILITY FOR ALL NatIVE HAWAIIAN PLANts Lucas Fortini1,2, Jonathan Price3, James Jacobi2, Adam Vorsino4, Jeff Burgett1,4, Kevin Brinck5, Fred Amidon4, Steve Miller4, Sam `Ohukani`ohi`a Gon III6, Gregory Koob7, and Eben Paxton2 1 Pacific Islands Climate Change Cooperative, Honolulu, HI 96813 2 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Hawaii National Park, HI 96718 3 Department of Geography & Environmental Studies, University of Hawai‘i at Hilo, Hilo, HI 96720 4 U.S. Fish & Wildlife Service —Ecological Services, Division of Climate Change and Strategic Habitat Management, Honolulu, HI 96850 5 Hawai‘i Cooperative Studies Unit, Pacific Island Ecosystems Research Center, Hawai‘i National Park, HI 96718 6 The Nature Conservancy, Hawai‘i Chapter, Honolulu, HI 96817 7 USDA Natural Resources Conservation Service, Hawaii/Pacific Islands Area State Office, Honolulu, HI 96850 Hawai‘i Cooperative Studies Unit University of Hawai‘i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 November 2013 This product was prepared under Cooperative Agreement CAG09AC00070 for the Pacific Island Ecosystems Research Center of the U.S. Geological Survey. Technical Report HCSU-044 A LANDSCAPE-BASED ASSESSMENT OF CLIMATE CHANGE VULNERABILITY FOR ALL NATIVE HAWAIIAN PLANTS LUCAS FORTINI1,2, JONATHAN PRICE3, JAMES JACOBI2, ADAM VORSINO4, JEFF BURGETT1,4, KEVIN BRINCK5, FRED AMIDON4, STEVE MILLER4, SAM ʽOHUKANIʽOHIʽA GON III 6, GREGORY KOOB7, AND EBEN PAXTON2 1 Pacific Islands Climate Change Cooperative, Honolulu, HI 96813 2 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Hawaiʽi National Park, HI 96718 3 Department of Geography & Environmental Studies, University of Hawaiʽi at Hilo, Hilo, HI 96720 4 U. -

Evaluating the Core Microbiome of Manduca Sexta Authors: Macy Johnson, Dr
Evaluating the Core Microbiome of Manduca sexta Authors: Macy Johnson, Dr. Jerreme Jackson*, and Dr. Tyrrell Conway† Abstract: Microbiomes are complex communities of microorganisms that colonize many surfaces of an animal’s body, especially those niches lined with carbohydrate-rich mucosal layers such as the eyes, male and female reproductive tracts, and the gastrointestinal tract. While a vast majority of data from microbiome studies has relied almost extensively on metagenomics-based approaches to identify individual species within these small complex communities, the contributions of these communities to host physiology remain poorly understood. We used a combination of culture- and non culture-based approaches to identify and begin functionally characterizing microbial inhabitants stably colonized in the midgut epithelium of the invertebrate model Manduca sexta (tobacco hornworm), an agriculture pest of Nicotiana attenuata (wild-tobacco) and many additional solanaceous plants. Keywords: Microbiome, Manduca sexta, Intestine, Nicotiana attenuata, Metagenomics Introduction supports the hypothesis that a core microbiome The animal intestinal microbiome comprises a persists in the intestinal tract of some Lepidopteran diverse community of microorganisms, which species. When the bacteria are transferred vertically, influence host development, physiology, and response they are passed on generationally. When the bacteria to pathogens. However, the mechanism underlying are transferred horizontally, they are passed directly these complex interactions -

Bats and Moths Contribute to the Reproductive Success of the Columnar Cactus Pilosocereus Leucocephalus
Journal of Arid Environments xxx (xxxx) xxxx Contents lists available at ScienceDirect Journal of Arid Environments journal homepage: www.elsevier.com/locate/jaridenv Bats and moths contribute to the reproductive success of the columnar cactus Pilosocereus leucocephalus ∗ Antonio Miranda-Jácomea, Ricardo Rodríguez-Garcíaa, Miguel A. Munguía-Rosasb, a Instituto de Investigaciones Biológicas, Universidad Veracruzana, Xalapa, 91190, Mexico b Laboratorio de Ecología Terrestre, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional (Cinvestav), Carretera Antigua a Progreso km 6, Mérida, 97310, Mexico ARTICLE INFO ABSTRACT Keywords: The pollination systems of columnar cacti in the dry tropics are often thought to be highly specialized to bats. Bat pollination This specialization is generally inferred when flowers that are only exposed to the activity of nocturnal visitors Columnar cactus set fruit and seed. Although moths are also common visitors to the flowers of columnar cacti at night, it is Moth pollination generally thought that their contribution to the reproductive success of this cactus is negligible. Using selective Pollination exclusions, we assessed the contribution of bats and moths to the reproductive success in a population of Pollination system Pilosocereus leucocephalus in central Mexico. Fruit set was 100% for bat-pollinated flowers and 34% in moth- pollinated flowers. Seed number per fruit was 1473 in bat-pollinated and 836 in moth pollinated flowers. Our results clearly show that in addition to bats, moths are effective pollinators of Pilosocereus leucocephalus in the study area. Therefore, bats are the main pollinators of P. leucocephalus, and moths are the secondary pollinators. Columnar cacti are the dominant elements in the plant communities fruit (e.g.