Gy Total Body Irradiation Followed by Allogeneic Hematopoietic St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Outcome of FLAG-IDA Chemotherapy Sequential
Bone Marrow Transplantation (2019) 54:458–464 https://doi.org/10.1038/s41409-018-0283-5 ARTICLE Clinical outcome of FLAG-IDA chemotherapy sequential with Flu–Bu3 conditioning regimen in patients with refractory AML: a parallel study from Shanghai Institute of Hematology and Institut Paoli-Calmettes 1 2 3 2 3 2 1 Ling Wang ● Raynier Devillier ● Ming Wan ● Justine Decroocq ● Liang Tian ● Sabine Fürst ● Li-Ning Wang ● 2 1 2 1 Norbert Vey ● Xing Fan ● Didier Blaise ● Jiong Hu Received: 23 January 2018 / Revised: 28 June 2018 / Accepted: 30 June 2018 / Published online: 6 August 2018 © The Author(s) 2018. This article is published with open access Abstract The purpose of the study was to evaluate the feasibility of conditioning regimen with sequential chemotherapy (FLAG-IDA), followed by Fludarabine (5 days) + Busulfan (3 days) by parallel analysis of patients with refractory acute myeloid leukemia (AML) from two transplantation centers in China and France. A total of 47 refractory AML with median bone marrow blast of 35% (1–90%) and median age at 42 years (16–62) were enrolled. Thirteen patients received peripheral stem cell 1234567890();,: 1234567890();,: transplantation (HSCT) from HLA-matched sibling donor, while 18 and 16 from unrelated or haplo-identical donors, respectively. With a median follow-up of 24.3 months (1–70), 13 patients relapsed at a median time of 5.1 months (2.2–18.0) and 24 patients died due to relapse (n = 12) or non-relapsed mortality (NRM, n = 12). The estimated 3-year RR and NRM were 33.5 ± 5.7% and 25.7 ± 4.2%, respectively. -

CK0403, a 9‑Aminoacridine, Is a Potent Anti‑Cancer Agent in Human Breast Cancer Cells
MOLECULAR MEDICINE REPORTS 13: 933-938, 2016 CK0403, a 9‑aminoacridine, is a potent anti‑cancer agent in human breast cancer cells YUAN-WAN SUN1, KUEN-YUAN CHEN2, CHUL-HOON KWON3 and KUN-MING CHEN1 1Department of Biochemistry and Molecular Biology, College of Medicine, Pennsylvania State University, Hershey, PA 17033, USA; 2Department of Surgery, National Taiwan University Hospital, Taipei 8862, Taiwan, R.O.C.; 3Department of Pharmaceutical Sciences, College of Pharmacy and Allied Health Professions, St. John's University, New York, NY 11439, USA Received November 11, 2014; Accepted October 22, 2015 DOI: 10.3892/mmr.2015.4604 Abstract. 3-({4-[4-(Acridin-9-ylamino)phenylthio]phenyl} reduced growth inhibitory activity under hypoxic conditions, (3-hydroxypropyl)amino)propan-1-ol (CK0403) is a which can induce autophagy. Collectively, the present results sulfur-containing 9-anilinoacridine analogue of amsacrine and supported that CK0403 is highly potent and more effective than was found to be more potent than its analogue 2-({4-[4-(acridin- CK0402 against estrogen receptor-negative and 9-ylamino)phenylthio]phenyl}(2-hydroxyethyl)amino) HER2-overexpressing breast cancer cell lines, suggesting its ethan-1-ol (CK0402) and amsacrine in the inhibition of the future application for chemotherapy in breast cancer. topoisomerase II-catalyzed decatenation reaction. A previous study by our group reported that CK0402 was effective against Introduction numerous breast cancer cell lines, and the combination of CK0402 with herceptin enhanced its activity in HER2(+) Breast cancer is the most common type of cancer diagnosed SKBR-3 cells. In order to identify novel chemotherapeutic agents in American women and is the second most common cause with enhanced potency, the present study explored the potential of cancer-associated mortality. -

Clinical Pharmacology of Antiмcancer Agents
A CLINI CLINICAL PHARMACOLOGY OF NTI- C ANTI-CANCER AGENTS C AL PHARMA AN Cristiana Sessa, Luca Gianni, Marina Garassino, Henk van Halteren www.esmo.org C ER A This is a user-friendly handbook, designed for young GENTS medical oncologists, where they can access the essential C information they need for providing the treatment which could OLOGY OF have the highest chance of being effective and tolerable. By finding out more about pharmacology from this handbook, young medical oncologists will be better able to assess the different options available and become more knowledgeable in the evaluation of new treatments. ESMO ESMO Handbook Series European Society for Medical Oncology CLINICAL PHARMACOLOGY OF Via Luigi Taddei 4, 6962 Viganello-Lugano, Switzerland Handbook Series ANTI-CANCER AGENTS Cristiana Sessa, Luca Gianni, Marina Garassino, Henk van Halteren ESMO Press · ISBN 978-88-906359-1-5 ISBN 978-88-906359-1-5 www.esmo.org 9 788890 635915 ESMO Handbook Series handbook_print_NEW.indd 1 30/08/12 16:32 ESMO HANDBOOK OF CLINICAL PHARMACOLOGY OF ANTI-CANCER AGENTS CM15 ESMO Handbook 2012 V14.indd1 1 2/9/12 17:17:35 ESMO HANDBOOK OF CLINICAL PHARMACOLOGY OF ANTI-CANCER AGENTS Edited by Cristiana Sessa Oncology Institute of Southern Switzerland, Bellinzona, Switzerland; Ospedale San Raffaele, IRCCS, Unit of New Drugs and Innovative Therapies, Department of Medical Oncology, Milan, Italy Luca Gianni Ospedale San Raffaele, IRCCS, Unit of New Drugs and Innovative Therapies, Department of Medical Oncology, Milan, Italy Marina Garassino Oncology Department, Istituto Nazionale dei Tumori, Milan, Italy Henk van Halteren Department of Internal Medicine, Gelderse Vallei Hospital, Ede, The Netherlands ESMO Press CM15 ESMO Handbook 2012 V14.indd3 3 2/9/12 17:17:35 First published in 2012 by ESMO Press © 2012 European Society for Medical Oncology All rights reserved. -

Sequential Combination of Decitabine
Li et al. Journal of Translational Medicine 2014, 12:167 http://www.translational-medicine.com/content/12/1/167 RESEARCH Open Access Sequential combination of decitabine and idarubicin synergistically enhances anti-leukemia effect followed by demethylating Wnt pathway inhibitor promoters and downregulating Wnt pathway nuclear target Kongfei Li1,2,3, Chao Hu1,3, Chen Mei6, Zhigang Ren4, Juan Carlos Vera5, Zhengping Zhuang5, Jie Jin1,3 and Hongyan Tong1,3* Abstract Background: The methylation inhibitor 5-Aza-2′-deoxycytidine (decitabine, DAC) has a great therapeutic value for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). But decitabine monotherapy was associated with a relatively low rate of complete remission in AML and MDS. We aimed to investigate the effect of several anti-leukemia drugs in combination with decitabine on the proliferation of myeloid leukemia cells, to select the most efficient combination group and explore the associated mechanisms of these combination therapies. Methods: Cell proliferation was tested by MTT assay and CFU-GM assay. Cell apoptosis was evaluated by Annexin V and PI staining in cell culture, TUNEL assay and transmission electron microscopy in animal study. MicroPET was used to imaging the tumor in mouse model. Molecular studies were conducted using microarray expression analysis, which was used to explore associated pathways, and real-time quantitative reverse transcription-PCR, western blot and immunohistochemistry, used to assess regulation of Wnt/β-catenin pathway. Statistical significance among groups was determined by one-way ANOVA analysis followed by post hoc Bonferroni’s multiple comparison test. Results: Among five anti-leukemia agents in combining with decitabine, the sequential combination of decitabine and idarubicin induced synergistic cell death in U937 cells, and this effect was verified in HEL, SKM-1 cells and AML cells isolated from AML patients. -

(Idarubicin) with Pharmacokinetic and in Vitro Drug Sensitivity Testing in Children with Refractory Leukemia1
(CANCER RESEARCH 48, 5348-5352. September 15. 1988] Phase I Clinical Trial of Orally Administered 4-Demethoxydaunorubicin (Idarubicin) with Pharmacokinetic and in Vitro Drug Sensitivity Testing in Children with Refractory Leukemia1 Ching-Hon Pui,2 Siebold S. N. de Graaf,3 Lois W. Dow, John H. Rodman, William E. Evans, Bruce S. Alpert, and Sharon B. Murphy Departments ofHematology and Oncology [C-H. P., L. W. D., S. B. M.] and Pharmaceutical Division [S. S. N. d. G., J. H. R., W. E. E.], St. Jude Children's Research Hospital, and Divisions of Hematology-Oncology [C-H. P., L. W. D., S. B. M.] and Cardiology [B. S. A.], Department of Pediatrics, University of Tennessee, Memphis, College of Medicine, Memphis, Tennessee 38101 ABSTRACT than that of analogues or i.v. idarubicin (9, 16). However, nausea and vomiting were generally more severe in patients Fifteen children with acute leukemia in relapse, refractory to conven receiving oral idarubicin (16). As a single agent in adults, oral tional therapy, were treated with idarubicin administered orally for 3 consecutive days in dosages ranging from 30 to 50 mg/m2 per day at 19- idarubicin has antileukemic activity at dosages of 45 to 90 mg/ m2 distributed over 3 consecutive days (9, 17, 18). Very little to 21-day intervals. Gastrointestinal complications, including nausea, vomiting, abdominal pain, diarrhea and stomatitis, were the major forms information is available for oral idarubicin treatment in children of dose-limiting toxicity, affecting the majority of patients at all levels of with leukemia. We report here the results of Phase I clinical, idarubicin dosage. -

FLAG-Idarubicin and Allogeneic Stem Cell Transplantation for Ph-Positive ALL Beyond first Remission
Bone Marrow Transplantation, (1998) 22, 1137–1143 1998 Stockton Press All rights reserved 0268–3369/98 $12.00 http://www.stockton-press.co.uk/bmt FLAG-idarubicin and allogeneic stem cell transplantation for Ph-positive ALL beyond first remission M Deane1,2, M Koh1, L Foroni1, G Galactowicz1, AV Hoffbrand1, M Lawler3, L Secker-Walker1 and HG Prentice1 2Department of Haematology, Norfolk and Norwich Hospital, Norwich, UK; 1Department of Haematology, Royal Free Hospital, London, UK; and 3Department of Haematology, Sir Patrick Dun Research Laboratory, St James’s Hospital, Dublin, Republic of Ireland Summary: median CR duration of 7 months and 13% 3-year disease- free survival (DFS) probability in adults.1,2,4 BMT from a We describe a single centre experience of eight consecu- sibling donor is a more effective treatment with 2-year DFS tive patients with relapsed or refractory Ph+ ALL probabilities of 38 and 46% for patients in 1st CR and 41 treated with the FLAG/idarubicin regimen followed by and 28% for patients beyond 1st CR in the IBMTR series5 BMT or PBSCT. Following FLAG/idarubicin, one achi- and the series reported by Chao et al.6 Radich et al7 eved a partial response and seven CR. All patients sub- reported 30 patients who received BMT in CR or relapse, sequently received allogeneic transplants: one sibling of whom nine were disease-free beyond 2 years. Casper et BMT, three matched unrelated (MUD) BMT and four al8 reported 68% DFS at 13 months in 14 paediatric sibling PBSCT. Two patients received second trans- patients who received BMT in CR. -
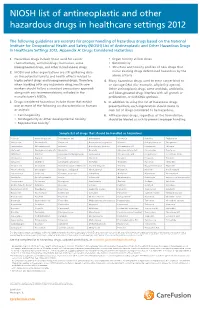
NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012
NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2012 The following guidelines are excerpts for proper handling of hazardous drugs based on the National Institute for Occupational Health and Safety (NIOSH) List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012, Appendix A: Drugs Considered Hazardous 1. Hazardous drugs include those used for cancer • Organ toxicity at low doses* chemotherapy, antiviral drugs, hormones, some • Genotoxicity** bioengineered drugs, and other miscellaneous drugs. • Structure and toxicity profiles of new drugs that 2. NIOSH and other organizations are still gathering data mimic existing drugs determined hazardous by the on the potential toxicity and health effects related to above criteria highly potent drugs and bioengineered drugs. Therefore, 4. Many hazardous drugs used to treat cancer bind to when working with any hazardous drug, health care or damage DNA (for example, alkylating agents). workers should follow a standard precautions approach Other antineoplastic drugs, some antivirals, antibiotics, along with any recommendations included in the and bioengineered drugs interfere with cell growth or manufacturer’s MSDSs. proliferation, or with DNA synthesis. 3. Drugs considered hazardous include those that exhibit 5. In addition to using the list of hazardous drugs one or more of the following six characteristics in humans presented here, each organization should create its or animals: own list of drugs considered to be hazardous. • Carcinogenicity 6. All hazardous -

Associated with Busulfan Clinical Pharmacology
PHARMACOKINETIC, PHARMACODYNAMIC AND PHARMACOGENETIC FACTORS ASSOCIATED WITH BUSULFAN CLINICAL PHARMACOLOGY PARTH J. UPADHYAY A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy School of Pharmacy Faculty of Health and Medicine The University of Sydney December 2018 | ॐ श्री गणेशाय नमः| For Bharat Bansilal Trivedi I hereby declare that this submission is my own work and to the best of my knowledge it contains no material previously published or written by another person, nor material which to a substantial extent has been accepted for the award of any degree or diploma at The University of Sydney or any other institution, except where due acknowledgement is made in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in this project’s design and conception or in style, presentation and linguistic expression is acknowledged. …………………………………………….. Parth J. Upadhyay January 2019 Table of Contents Pharmacokinetic, Pharmacodynamic and Pharmacogenetic Factors Associated with Busulfan Clinical Pharmacology ........................................................................... 1 Acknowledgements ........................................................................................................... vii Presentations Arising From This Thesis ............................................................................ xii Abstract xv Abbreviations ................................................................................................................. -

Relapsed Acute Myeloblastic Leukemia: First Pediatric Randomized Study
Editorial Relapsed acute myeloblastic leukemia: first pediatric randomized study Jan Styczynski Department of Pediatric Hematology and Oncology, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland Correspondence to: Jan Styczynski, MD, PhD. Department of Pediatric Hematology and Oncology, Collegium Medicum, Nicolaus Copernicus University, ul. Curie-Sklodowskiej 9, 85-094 Bydgoszcz, Poland. Email: [email protected]. Submitted Apr 02, 2013. Accepted for publication Apr 22, 2013. doi: 10.3978/j.issn.2224-4336.2013.04.05 View this article at: http://www.thetp.org/article/view/1833/2590 The current cure rate achieves 80% of long-term survival DNX and 70% for patients randomly assigned to FLAG. in childhood acute lymphoblastic leukemia (ALL) and only Concerning secondary end points, the CR rate was 69% 50-60% in acute myeloblastic leukemia (AML) (1). with FLAG/DNX and 59% with FLAG, but overall However, in patients who relapse following treatment survival was similar. However, in the subgroup of patients, of AML, the odds are not as far in their favor, and the with core-binding factor (CBF) AML treated with FLAG/ probability of overall survival is 16-34% (2,3). DNX resulted in pOS of 82% versus 58% with FLAG, In a recent manuscript published in the Journal of Clinical what is a unique result of the study. Another important Oncology, Kaspers et al. (4), report results of a randomized issue is similar grade 3/4 toxicity in both groups. Kaspers phase III study by the International Berlin-Frankfurt- et al. concluded, that DNX added to FLAG improves early Münster Study Group in pediatric relapsed AML, with treatment response in pediatric relapsed AML. -

Intercalating TOP2 Poisons Attenuate Topoisomerase Action at Higher Concentrations
Molecular Pharmacology Fast Forward. Published on August 9, 2019 as DOI: 10.1124/mol.119.117259 This article has not been copyedited and formatted. The final version may differ from this version. Intercalating TOP2 poisons attenuate topoisomerase action at higher concentrations Mandeep Atwal, Rebecca L. Swan, Chloe Rowe, Ka C. Lee, David C Lee, Lyle Armstrong, Ian G Cowell*, Caroline A Austin* Running title: Inhibition by doxorubicin, epirubicin and mitoxantrone MA, RLS, CR, KCL, IGC, CAA Institute for Cell and Molecular Biosciences, Newcastle University, Newcastle upon Tyne. NE2 4HH. U.K. Downloaded from DCL, LA Institute of Genetic Medicine, Newcastle University, International Centre for Life, Central Parkway, Newcastle upon Tyne. NE1 3BZ. DCL: https://orcid.org/0000-0001-6857-624X IGC: https://orcid.org/0000-0002-8606-0447 molpharm.aspetjournals.org CAA: https://orcid.org/0000-0002-1921-5947 *Corresponding authors: Ian G Cowell & Caroline A Austin Institute for Cell and Molecular Biosciences, Newcastle University, Newcastle upon Tyne NE2 4HH. United Kingdom at ASPET Journals on September 26, 2021 Tel: +44 (0)191 208 7677 Fax: +44 (0)191 208 7424 Email: [email protected], [email protected] 30 text pages (including refs and figure legends) 8 figures 0 tables 60 number of references 219 words in abstract 641 words in Introduction 1268 words in Discussion Abbreviations DMSO Dimethyl sulfoxide DOX Doxorubicin DSB Double-strand break EPI Epirubicin ICRF-193 4-[2-(3,5-Dioxo-1-piperazinyl)-1-methylpropyl]piperazine-2,6-dione TARDIS Trapped in Agarose DNA Immunostaining assay TOP2 Topoisomerase II TOP2A Topoisomerase IIα TOP2B Topoisomerase IIβ TOP2-CC Topoisomerase II covalent complex 1 Molecular Pharmacology Fast Forward. -

Acute Myeloid Leukemia
Kantarjian et al. Blood Cancer Journal (2021) 11:41 https://doi.org/10.1038/s41408-021-00425-3 Blood Cancer Journal REVIEW ARTICLE Open Access Acute myeloid leukemia: current progress and future directions Hagop Kantarjian 1, Tapan Kadia 1, Courtney DiNardo 1, Naval Daver 1, Gautam Borthakur 1, Elias Jabbour1, Guillermo Garcia-Manero 1, Marina Konopleva 1 and Farhad Ravandi1 Abstract Progress in the understanding of the biology and therapy of acute myeloid leukemia (AML) is occurring rapidly. Since 2017, nine agents have been approved for various indications in AML. These included several targeted therapies like venetoclax, FLT3 inhibitors, IDH inhibitors, and others. The management of AML is complicated, highlighting the need for expertise in order to deliver optimal therapy and achieve optimal outcomes. The multiple subentities in AML require very different therapies. In this review, we summarize the important pathophysiologies driving AML, review current therapies in standard practice, and address present and future research directions. Introduction regimens consisting of all trans-retinoic acid (ATRA) and Progress in understanding the pathophysiology and arsenic trioxide in acute promyelocytic leukemia (APL) – improving the therapy of acute myeloid leukemia (AML) resulted in cure rates of 90%8 12. In core-binding factor is now occurring at a rapid pace. The discovery of the (CBF) AML, adding gemtuzumab ozogamicin (CD33-tar- 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; activity of cytarabine (ara-C) and of anthracyclines in geted monoclonal antibody conjugated to the calicheamicin AML, and combining them in the 1970’s, into what is payload) to high-dose cytarabine-based chemotherapy – known as the “3 + 7 regimen” (3 days of daunorubicin + increased the long-term survival rate from 50% to 75+%13 17. -

An Insight Into Cancer and Anticancer Drugs
ACTA SCIENTIFIC MEDICAL SCIENCES (ISSN: 2582-0931) Volume 3 Issue 8 August 2019 Review Article An Insight into Cancer and Anticancer Drugs X Raichel Nivetha1, M Jeslin Jeba Soundari1, MSA Muthukumar Nadar1, D Premnath1, Paulraj Mosae Selvakumar2* and Ruey-yi Chang3 1Department of Biotechnology, Karunya Institute of Technology and Sciences (Deemed to be University), Coimbatore, Tamilnadu, India 2Science and Math Program, Asian University for Women, Chittagong, Bangladesh 3Department of Life Sciences, National Dong Hwa University, Hualien, Taiwan, China *Corresponding Author: Paulraj Mosae Selvakumar, Science and Math program, Asian university for women, Chittagong, Bangladesh. Received: June 03, 2019; Published: July 04, 2019 DOI: 10.31080/ASMS.2019.03.0340 Abstract Cancer is one of the most common and widespread diseases in the human population and has been reported to be the second worldwide, each of which requires unique approaches for treatment. It also records that 1 in every 6 deaths in the world are due to major cause of death globally. The World Health Organisation states that about 200 different types of cancers have been identified cancer which clearly evidences for the inevitable necessity for the control and cure of cancer. Unlike normal cells, cancer cells un- dergo uncontrollable cell division and each division leads to multiple mutations in a continuous fashion. There are various strategies towards targeting cancerous cells. With the advent of conventional therapies, various chemical derivatives are being used as anti- designing by identifying potent targets. Here we will be discussing about the history of cancer and its advances towards development cancer drugs. This article reviews on the various approaches put forth in the fight against cancer through cancer therapies and drug of anticancer drugs from tip to toe.