Prader–Willi Syndrome and Hypogonadism: a Review Article
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Background Briefing Document from the Consortium of Sponsors for The
BRIEFING BOOK FOR Pediatric Advisory Committee (PAC) Prepared by Alan Rogol, M.D., Ph.D. Prepared for Consortium of Sponsors Meeting Date: April 8th, 2019 TABLE OF CONTENTS LIST OF FIGURES ................................................... ERROR! BOOKMARK NOT DEFINED. LIST OF TABLES ...........................................................................................................................4 1. INTRODUCTION AND BACKGROUND FOR THE MEETING .............................6 1.1. INDICATION AND USAGE .......................................................................................6 2. SPONSOR CONSORTIUM PARTICIPANTS ............................................................6 2.1. TIMELINE FOR SPONSOR ENGAGEMENT FOR PEDIATRIC ADVISORY COMMITTEE (PAC): ............................................................................6 3. BACKGROUND AND RATIONALE .........................................................................7 3.1. INTRODUCTION ........................................................................................................7 3.2. PHYSICAL CHANGES OF PUBERTY ......................................................................7 3.2.1. Boys ..............................................................................................................................7 3.2.2. Growth and Pubertal Development ..............................................................................8 3.3. AGE AT ONSET OF PUBERTY.................................................................................9 3.4. -
Case Report Full Text Online At
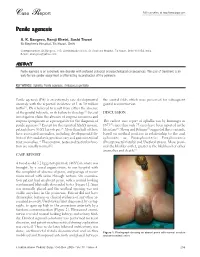
Case Report Full text online at http://www.jiaps.com Penile agenesis A. K. Bangroo, Ramji Khetri, Sashi Tiwari St Stephen's Hospital, Tis Hazari, Delhi Correspondence: AK Bangroo, 103, Administrative block, St. Stephens Hospital, Tis Hazari, Delhi-110054, India. E-mail: [email protected] ABSTRACT Penile agenesis is an extremely rare disorder with profound urological and psychological consequences. The goal of treatment is an early female gender assignment and feminizing reconstruction of the perineum. KEY WORDS: Aphallia, Penile agenesis, Ambiguous genitalia Penile agenesis (PA) is an extremely rare developmental the scrotal folds which were preserved for subsequent anomaly with the reported incidence of 1 in 30 million genital reconstruction. births[1]. PA is believed to result from either the absence of the genital tubercle, or its failure to develop.[2] Several DISCUSSION investigators claim the absence of corpora cavernosa and corpora spongiosum as a prerequisite for the diagnosis of The earliest case report of aphallia was by Imminger in penile agenesis.[3] Except for the reported XX-XY mosaic, 1853[2] since then only 75 cases have been reported in the patients have 46 XY karyotypes.[4] More than half of these literature[6]. Skoog and Belman[5] suggested three variants, have associated anomalies, including developmental de based on urethral position in relationship to the anal fects of the caudal axis, genitourinary and gastrointestinal sphincter, as: Postsphincteric; Presphincteric tract anomalies.[5] The scrotum, testes and testicular func (Prostatorectal fistula) and Urethral atresia. More proxi tion are usually normal[2]. mal the bladder outlet, greater is the likelihood of other anomalies and death.[5] CASE REPORT A two-day-old 3.2 kg genotypic male (46XY) neonate was brought, by a social organization, to our hospital with the complaint of absence of penis, and passage of meco nium mixed with urine through rectum. -

EAU Pocket Guidelines on Male Hypogonadism 2013
GUIDELINES ON MALE HYPOGONADISM G.R. Dohle (chair), S. Arver, C. Bettocchi, S. Kliesch, M. Punab, W. de Ronde Introduction Male hypogonadism is a clinical syndrome caused by andro- gen deficiency. It may adversely affect multiple organ func- tions and quality of life. Androgens play a crucial role in the development and maintenance of male reproductive and sexual functions. Low levels of circulating androgens can cause disturbances in male sexual development, resulting in congenital abnormalities of the male reproductive tract. Later in life, this may cause reduced fertility, sexual dysfunc- tion, decreased muscle formation and bone mineralisation, disturbances of fat metabolism, and cognitive dysfunction. Testosterone levels decrease as a process of ageing: signs and symptoms caused by this decline can be considered a normal part of ageing. However, low testosterone levels are also associated with several chronic diseases, and sympto- matic patients may benefit from testosterone treatment. Androgen deficiency increases with age; an annual decline in circulating testosterone of 0.4-2.0% has been reported. In middle-aged men, the incidence was found to be 6%. It is more prevalent in older men, in men with obesity, those with co-morbidities, and in men with a poor health status. Aetiology and forms Male hypogonadism can be classified in 4 forms: 1. Primary forms caused by testicular insufficiency. 2. Secondary forms caused by hypothalamic-pituitary dysfunction. 164 Male Hypogonadism 3. Late onset hypogonadism. 4. Male hypogonadism due to androgen receptor insensitivity. The main causes of these different forms of hypogonadism are highlighted in Table 1. The type of hypogonadism has to be differentiated, as this has implications for patient evaluation and treatment and enables identification of patients with associated health problems. -

A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years
Journal of Advances in Radiology and Medical Imaging Volume 4 | Issue 1 ISSN: 2456-5504 Case Report Open Access A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man D’Amato D*, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, Valente F and Bizzaglia M Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Rome, Italy *Corresponding author: D’Amato D, Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Viale Oxford 181, Rome, Italy, Tel: +393207034690, E-mail: [email protected] Citation: D’Amato D, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, et al. (2019) A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man. J Adv Radiol Med Image 4(1): 102 Received Date: April 04, 2019 Accepted Date: August 26, 2019 Published Date: August 28, 2019 Abstract Congenital anorchia is an uncommon clinical condition. Etiology and pathogenetic mechanisms are often unknown. Although some patients with anorchia present with ambiguous external genitalia or micropenis, most have a normal phenotype. XY Disorders of Sex Development classifications are numerous and success rate in establishing a precise diagnosis is far lower than in XX karyotype. We report the case of a young man, with 46 XY karyotype showing various uro-genital abnormalities. A definitive diagnosis was not established due to the complex clinical presentation. Ultrasonography and Magnetic Resonance Imaging techniques were useful tools in the definition of uro-genital anomalies and gonadal development in this complex scenario. Keywords: Anorchia; Cryptorchidism; Urogenital Anomalies; DSD; MRI List of abbreviations: MRI: Magnetic Resonance Imaging; US: Ultrasonography; DSD: Disorders of Sex Development, FSH: Follicle- Stimulating Hormone; HCG: Human Chorionic Gonadotropin; LH: Luteinizing Hormone; AMH Antimüllerian Hormone; LHRH LH- Releasing Hormone; SD: Standard Deviation Introduction The disorders of sexual differentiation constitute a challenging area for both diagnostic and therapeutic impact. -

Treatment of Peripheral Precocious Puberty
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by IUPUIScholarWorks Treatment of Peripheral Precocious Puberty Melissa Schoelwer, MD and Erica A Eugster, MD Section of Pediatric Endocrinology, Department of Pediatrics, Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, Indiana Send correspondence to: 705 Riley Hospital Drive, Room 5960 Indianapolis, IN 46202 Phone: 317-944-3889 Fax: 317-944-3882 Email: [email protected] __________________________________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Schoelwer, M., & Eugster, E. A. (2016). Treatment of Peripheral Precocious Puberty. In Puberty from Bench to Clinic (Vol. 29, pp. 230-239). Karger Publishers. http://dx.doi.org/10.1159/000438895 Peripheral Precocious Puberty Abstract There are many etiologies of peripheral precocious puberty (PPP) with diverse manifestations resulting from exposure to androgens, estrogens, or both. The clinical presentation depends on the underlying process and may be acute or gradual. The primary goals of therapy are to halt pubertal development and restore sex steroids to prepubertal values. Attenuation of linear growth velocity and rate of skeletal maturation in order to maximize height potential are additional considerations for many patients. McCune-Albright syndrome (MAS) and Familial Male-Limited Precocious Puberty (FMPP) represent rare causes of PPP that arise from activating mutations in GNAS1 and the LH receptor gene, respectively. Several different therapeutic approaches have been investigated for both conditions with variable success. Experience to date suggests that the ideal therapy for precocious puberty secondary to MAS in girls remains elusive. In contrast, while the number of treated patients remains small, several successful therapeutic options for FMPP are available. -

Precocious Puberty Children with Spina Bi�Da and Hydrocephalus May Start Puberty Earlier Than Their Peers
SBA National Resource Center: 800-621-3141 Precocious Puberty Children with Spina Bida and hydrocephalus may start puberty earlier than their peers. What is Puberty? If major breast development starts before age 8, it is considered early. (Sometimes girls will have some Puberty refers to normal body changes that lead to breast development, with no other signs of puberty. maturity and the ability to have children. Normal puberty This isolated change may be normal.) begins between ages 8 and 12 in girls and between 9 and 14 in boys. Hormones made in the brain control the timing and sequence of puberty. These hormones What are the stages of normal puberty in boys? stimulate other parts of the body to make sex hormones. The usual sequence in boys is: The sex hormones, especially estrogen in girls and testosterone in boys, cause sexual maturation. • The testicles grow larger. • The penis grows larger. What are the stages of normal puberty in girls? • Pubic hair grows. The physical changes seen in puberty are labeled by “Tanner staging.” Stage 1 is child-like (before puberty) • There is a growth spurt.rt. and stage 5 is full maturity. The usual sequence in girls is: • Other body hair grows.s. • Breasts start to develop. If boys show major developmentelopment • Hips widen and a there is a growth spurt that usually before age 9, it is considereddered lasts about three to four years. early. Early puberty in girls or boys is called • Pubic hair grows (three-to-six months after breasts “Precocious Puberty.” develop). • Other body hair grows. -

Growth Hormone Deficiency Causing Micropenis:Peter A
Growth Hormone Deficiency Causing Micropenis:Peter A. Lee, MD, PhD, a Tom Mazur, PsyD, Lessons b Christopher P. Houk, MD, Learned c Robert M. Blizzard, MD d From a Well-Adjusted Adultabstract This report of a 46, XY patient born with a micropenis consistent with etiology from isolated congenital growth hormone deficiency is used to (1) raise the question regarding what degree testicular testosterone exposure aDepartment of Pediatrics, College of Medicine, Penn State to the central nervous system during fetal life and early infancy has on the University, Hershey, Pennsylvania; bCenter for Psychosexual development of male gender identity, regardless of gender of rearing; (2) Health, Jacobs School of Medicine and Biomedical suggest the obligatory nature of timely full disclosure of medical history; Sciences, University at Buffalo and John R. Oishei Children’s Hospital, Buffalo, New York; cDepartment of (3) emphasize that virtually all 46, XY infants with functional testes and Pediatrics, Medical College of Georgia, Augusta University, a micropenis should be initially boys except some with partial androgen Augusta, Georgia; and dDepartment of Pediatrics, College of Medicine, University of Virginia, Charlottesville, Virginia insensitivity syndrome; and (4) highlight the sustaining value of a positive long-term relationship with a trusted physician (R.M.B.). When this infant Dr Lee reviewed and discussed the extensive medical records with Dr Blizzard, reviewed presented, it was commonly considered inappropriate to gender assign an pertinent medical literature, and wrote each draft infant male whose penis was so small that an adult size was expected to be of the manuscript with input from all coauthors; inadequate, even if the karyotype was 46, XY, and testes were functional. -
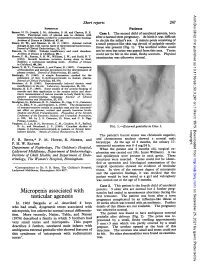
Micropenis Associated with Testicular Agenesis
Arch Dis Child: first published as 10.1136/adc.50.3.247 on 1 March 1975. Downloaded from Short reports 247 REFERENCES Patients Barnes, N. D., Joseph, J. M., Atherden, S. M. and Clayton, B. E. (1972). Functional tests of adrenal axis in children with Case 1. The second child of unrelated parents, born measurement of plasma cortisol by competitive protein binding. after a normal term pregnancy. At birth it was difficult Archives of Disease in Childhood, 47, 66. to decide the infant's sex. A minute penis consisting of Deane, H. W., and Masson, G. M. C. (1951). Adrenal cortical a small prepuce-like skin tag devoid of palpable erectile changes in rats with various types of experimental hypertension. .ournal of Clinical Endocrinology, 11, 193. tissue was present (Fig. 1). The urethral orifice could Fanconi, G. (1954). Tubular insufficiency and renal dwarfism. not be seen but urine was passed from this area. Testes Archives of Disease in Childhood, 29, 1. could not be felt in the small, fleshy scrotum. Physical Howse, P. M., Rayner, P. H. W., Williams, J. W., and Rudd, B. T. (1974). Growth hormone secretion during sleep in short examination was otherwise normal. children: a continuous sampling study. Archives of Disease in Childhood, 49, 246. James, V. H. T., Townsend, J., and Fraser, R. (1967). Comparison of fluorimetric and isotopic procedures for the determination of plasma cortisol. journal of Endocrinology, 37, xxviii. Mattingly, D. (1962). A simple fluorimetric method for the estimation of free 1 1-hydroxycorticoids in human plasma. Journal of Clinical Pathology, 15, 374. -

Precocious Puberty: a Red Flag for Malignancy in Childhood
CLINICAL Paul R. D’Alessandro, MD, MSc, Jillian Hamilton, MBChB, Karine Khatchadourian, MD, MSc, Ewa Lunaczek-Motyka, MD, Kirk R. Schultz, MD, Daniel Metzger, MD, Rebecca J. Deyell, MD, MHSc Precocious puberty: A red flag for malignancy in childhood Three clinical cases of precocious puberty resulting from rare but serious functional solid tumors in children highlight the need for physicians to identify the condition early and refer to tertiary care to minimize morbidity and optimize survival. ABSTRACT: Pediatric solid tumors have a range of 1-3 Dr D’Alessandro is a pediatric hematology/ abnormalities. These tumors may present clinical presentations, including those driven by oncology subspecialty resident in the with early onset of isosexual or contrasexual the ectopic production of hormones secreted by Division of Pediatric Hematology, Oncology, puberty as a first symptom. Treatment may some malignancies. Functional tumors lead to a and Bone Marrow Transplant, Department involve surgery, chemotherapy, and/or radio- variety of presentations, including Cushing syn- of Pediatrics, University of British therapy. Genetic testing or counseling may be drome, growth acceleration, abnormal virilization Columbia, British Columbia Children’s indicated for families. Early identification mini- or feminization, and hypertension with electrolyte Hospital Research Institute. mizes disease-related morbidity and mortality abnormalities. Precocious puberty, the onset of Dr Hamilton is a general practice specialty and optimizes outcomes. We present illustrative secondary sexual characteristics before age 8 in trainee, NHS Forth Valley, Larbert, cases of functional solid tumors in children from girls or 9 in boys, may be a warning sign of occult United Kingdom. Dr Khatchadourian is a British Columbia with the aim of educating malignancy. -

Precocious Puberty
Precocious Puberty The Pituitary Gland: The "Master Gland" The pituitary gland, which is often referred to as the "master gland", regulates the release of most of the body's hormones (chemical messengers that send information to different parts of the body). It is a pea-sized gland that is located underneath the brain. The pituitary gland controls the release of thyroid, adrenal, growth and sex hormones. The hypothalamus, located in the brain above the pituitary gland, regulates the release of hormones from the pituitary gland. Hormones: The "Chemical Messengers" Chemical messengers that carry information from one cell to another in the body. Hormones are carried throughout body by the blood, and are responsible for regulating many body functions. The body makes many hormones (e.g., thyroid, growth, sex and adrenal hormones) that work together to maintain normal bodily function. Hormones involved in the control of puberty include: GnRH: Gonadotropin releasing hormone, which comes from the brain in boys and in girls. Other androgens from the adrenal glands (located near the kidneys) produce pubic and axillary hair at the time of puberty. Estrogen: A female sex hormone, which is responsible for breast development in girls. It is made mainly by the ovaries, but is also present in boys in smaller amounts. Sex Hormones: Responsible for the development of pubertal signs as well as changes in behavior and the ability to have children. Precocious Puberty Precocious puberty means having signs of puberty (e.g., pubic hair or breast development) at an earlier age than usual. Normal Puberty There is a wide range of ages at which individuals normally start puberty. -

Intersex, Discrimination and the Healthcare Environment – a Critical Investigation of Current English Law
Intersex, Discrimination and the Healthcare Environment – a Critical Investigation of Current English Law Karen Jane Brown Submitted in Partial Fulfilment of the Requirements of London Metropolitan University for the Award of PhD Year of final Submission: 2016 Table of Contents Table of Contents......................................................................................................................i Table of Figures........................................................................................................................v Table of Abbreviations.............................................................................................................v Tables of Cases........................................................................................................................vi Domestic cases...vi Cases from the European Court of Human Rights...vii International Jurisprudence...vii Tables of Legislation.............................................................................................................viii Table of Statutes- England…viii Table of Statutory Instruments- England…x Table of Legislation-Scotland…x Table of European and International Measures...x Conventions...x Directives...x Table of Legislation-Australia...xi Table of Legislation-Germany...x Table of Legislation-Malta...x Table of Legislation-New Zealand...xi Table of Legislation-Republic of Ireland...x Table of Legislation-South Africa...xi Objectives of Thesis................................................................................................................xii -

Pathogenic Variants in the DEAH-Box RNA Helicase DHX37 Are a Frequent Cause of 46,XY Gonadal Dysgenesis and 46,XY Testicular Regression Syndrome
ARTICLE © American College of Medical Genetics and Genomics Pathogenic variants in the DEAH-box RNA helicase DHX37 are a frequent cause of 46,XY gonadal dysgenesis and 46,XY testicular regression syndrome Ken McElreavey, PhD 1, Anne Jorgensen, PhD 2, Caroline Eozenou, PhD1, Tiphanie Merel, MSc1, Joelle Bignon-Topalovic, BSc1, Daisylyn Senna Tan, BSc3, Denis Houzelstein, PhD 1, Federica Buonocore, PhD 4, Nick Warr, PhD 5, Raissa G. G. Kay, PhD5, Matthieu Peycelon, MD, PhD 6,7,8, Jean-Pierre Siffroi, MD, PhD6, Inas Mazen, MD9, John C. Achermann, MD, PhD 4, Yuliya Shcherbak, MD10, Juliane Leger, MD, PhD11, Agnes Sallai, MD 12, Jean-Claude Carel, MD, PhD 11, Laetitia Martinerie, MD, PhD11, Romain Le Ru, MD13, Gerard S. Conway, MD, PhD14, Brigitte Mignot, MD15, Lionel Van Maldergem, MD, PhD 16, Rita Bertalan, MD, PhD17, Evgenia Globa, MD, PhD 18, Raja Brauner, MD, PhD19, Ralf Jauch, PhD 3, Serge Nef, PhD 20, Andy Greenfield, PhD5 and Anu Bashamboo, PhD1 Purpose: XY individuals with disorders/differences of sex devel- specifically associated with gonadal dysgenesis and TRS. opment (DSD) are characterized by reduced androgenization Consistent with a role in early testis development, DHX37 is caused, in some children, by gonadal dysgenesis or testis regression expressed specifically in somatic cells of the developing human during fetal development. The genetic etiology for most patients and mouse testis. with 46,XY gonadal dysgenesis and for all patients with testicular Conclusion: DHX37 pathogenic variants are a new cause of an regression syndrome (TRS) is unknown. autosomal dominant form of 46,XY DSD, including gonadal Methods: We performed exome and/or Sanger sequencing in 145 dysgenesis and TRS, showing that these conditions are part of a individuals with 46,XY DSD of unknown etiology including clinical spectrum.