The Incubation of Ratite Eggs
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Incubation Constancy in the Red-Winged Blackbird
INCUBATION CONSTANCY IN THE RED-WINGED BLACKBIRD LARRY C. HOLCOMB Avian incubation behavior is affected by a multitude of exogenous and en- dogenous factors. Kendeigh (1952, 196313) and Skutch (1962) reviewed in- cubation in many different orders of birds and discussed factors affecting the amount of time spent in incubation. Among workers recently reporting on incubation behavior in wild passerines are Prescott (1964)) Mumford (1964)) Erpino (1968)) Maxwell and Putnam (1972)) and Morton et al. (1972). In the Red-winged Blackbird (Ag e1 aius phoeniceus) , Nero (1956a, 195613) has published observations on female behavior during the repro- ductive cycle, but nothing was reported on the incubation constancy (per- cent of daylight hours spent on the nest). I have reported (Holcomb, 1968, 1970) that female Redwings incubated normal-sized artificial eggs a mean of 19.4 days before abandoning them. This is 8.4 days beyond the normal incubation period. The present study was designed to determine the incubation constancy in the egg-laying period, to discover if it increased each day as the incubation behavior developed, and to find if there was less incubation each day as females neared the day when eggs were abandoned in prolonged incubation. METHODS I studied the incubation behavior of Red-winged Blackbirds near Omaha, Nebraska, in 1968 and 1969. Birds were breeding in a variety of habitats, including weed, alfalfa, and clover fields, hedgerows, ditch banks, and marshes. I visited the nesting areas nearly every day, beginning in March and ending in August. Male Redwings generally arrived in early March and females soon afterward. -

Care of Fertile Eggs Prior to Incubation 1
CARE OF FERTILE EGGS PRIOR TO INCUBATION 1. Keep eggs at 50º - 60º F (room temperature). (DON’T PUT IN A REFRIGERATOR, IT IS TOO COLD!) 2. Store the eggs with the BIG end up in egg cartons. 3. You can store for 10 days after they have been laid before hatch rate decreases (50% hatch rate is a good rate). Incubation / hatching time begins once the eggs are placed in the incubator and brought up to 99 ½ºF. Count day 1 after first 24 hours. Incubation/hatching time for chickens are 21 days and 28 to 33 days for ducks. INCUBATOR SET UP 1. Place the incubator(s) away from any windows as the sunlight will magnify thru the plexi-glass cover making it too hot. 2. Set up the incubator four (4) hours prior to adding eggs; it will give the incubator an opportunity to Diagram #2 Round Corner regulate the water temperature in the reservoir. Diagram #1 Put water into both of the troughs on the square Square corner incubator incubator cornered incubators (see diagram #1). In the round corner model which has a larger and smaller trough, place water in the outside trough labeled “circulating” (see diagram #2). (Be sure that the incubator turns on at 99º and off at 100ºF.) 3. Place the plastic thermometer, simply lay it across the top of the eggs, (see diagram #5). It will basically ride on top of the eggs. 4. Cover the adjusting stem on top with paper cup taped to help eliminate the likelihood of the setting being accidentally changed. -

Ratite Molecular Evolution, Phylogeny and Biogeography Inferred from Complete Mitochondrial Genomes
RATITE MOLECULAR EVOLUTION, PHYLOGENY AND BIOGEOGRAPHY INFERRED FROM COMPLETE MITOCHONDRIAL GENOMES by Oliver Haddrath A thesis submitted in confonnity with the requirements for the Degree of Masters of Science Graduate Department of Zoology University of Toronto O Copyright by Oliver Haddrath 2000 National Library Biblioth&que nationale 191 .,,da du Canada uisitions and Acquisitions et Services services bibliographiques 395 Welington Street 395. rue WdKngton Ottawa ON KIA ON4 Otîâwâ ON K1A ûN4 Canada Canada The author has granted a non- L'auteur a accordé une iicence non exclusive licence allowing the exclusive permettant A la National Library of Canada to Bihliotheque nationale du Canada de reproduce, loan, distribute or sell reproduire, @ter, distribuer ou copies of diis thesis in microfonn, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/fïîm, de reproduction sur papier ou sur format 61ectronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette tbése. thesis nor substantial exûacts fiom it Ni la thèse ni des extraits substantiels may be priated or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. Abstract Ratite Molecular Evolution, Phylogeny and Biogeography Inferred fiom Complete Mitochoncîrial Genomes. Masters of Science. 2000. Oliver Haddrath Department of Zoology, University of Toronto. The relationships within the ratite birds and their biogeographic history has been debated for over a century. While the monophyly of the ratites has been established, consensus on the branching pattern within the ratite tree has not yet been reached. -

Class Three: Breeding
Class Three: Breeding WHERE DO SEABIRDS LAY THEIR EGGS? Nest or no nest? • Some species use no nesting material. E.g., the white tern lays a single egg on an open branch. • Some species use a very little bit of nesting material. E.g., the tufted puffin may use a few pieces of grass and a couple of feathers. • Some species build more substantial nests. E.g. kittiwakes cement their nests onto small cliff shelves by trampling mud and guano to form a base. And, some cormorants build large nests in trees from sticks and twigs. [email protected] www.seabirdyouth.org 1 White tern • Also called fairy tern. • Tropical seabird species. • Lays egg on branch or fork in tree. No nest. • Newly hatched chicks have well developed feet to hang onto the nesting-site. White Tern. © Pillot, via Creative Commons. On the coast or inland? • Most seabird species breed on the coast and offshore islands. • Some species breed fairly far inland, but still commute to the ocean to feed. E.g., kittlitz’s murrelets nest on scree slopes on coastal mountains, and parents may travel more than 70km to their feeding grounds. • Other species breed far inland and never travel to the ocean. E.g., double crested cormorants breed on the coast, but also on lakes in many states such as Minnesota. [email protected] www.seabirdyouth.org 2 NESTING HABITAT (1) Ground Some species breed on the ground. These species tend to breed in areas with little or no predation, such as offshore islands (e.g., terns and gulls) or in the Antarctic (e.g., penguins, albatross). -

Chapter 02 Biogeography and Evolution in the Tropics
Chapter 02 Biogeography and Evolution in the Tropics (a) (b) PLATE 2-1 (a) Coquerel’s Sifaka (Propithecus coquereli), a lemur species common to low-elevation, dry deciduous forests in Madagascar. (b) Ring-tailed lemurs (Lemur catta) are highly social. PowerPoint Tips (Refer to the Microsoft Help feature for specific questions about PowerPoint. Copyright The Princeton University Press. Permission required for reproduction or display. FIGURE 2-1 This map shows the major biogeographic regions of the world. Each is distinct from the others because each has various endemic groups of plants and animals. FIGURE 2-2 Wallace’s Line was originally developed by Alfred Russel Wallace based on the distribution of animal groups. Those typical of tropical Asia occur on the west side of the line; those typical of Australia and New Guinea occur on the east side of the line. FIGURE 2-3 Examples of animals found on either side of Wallace’s Line. West of the line, nearer tropical Asia, one 3 nds species such as (a) proboscis monkey (Nasalis larvatus), (b) 3 ying lizard (Draco sp.), (c) Bornean bristlehead (Pityriasis gymnocephala). East of the line one 3 nds such species as (d) yellow-crested cockatoo (Cacatua sulphurea), (e) various tree kangaroos (Dendrolagus sp.), and (f) spotted cuscus (Spilocuscus maculates). Some of these species are either threatened or endangered. PLATE 2-2 These vertebrate animals are each endemic to the Galápagos Islands, but each traces its ancestry to animals living in South America. (a) and (b) Galápagos tortoise (Geochelone nigra). These two images show (a) a saddle-shelled tortoise and (b) a dome-shelled tortoise. -

Egg Laying in Pet Birds Egg Laying in Pet Birds Can Be a Serious Health Threat
Egg Laying in Pet Birds Egg laying in pet birds can be a serious health threat. This article explains why (and what to do if) your bird starts laying eggs. Providing proper, non-incandescent lighting, a healthy diet, and adequate sleep, as well as removing nesting toys or materials are key to discouraging egg laying. Overview In wild birds and breeding birds, egg laying is a natural, seasonal process. However, female pet birds can also lay eggs, even without the presence of a male. Such eggs are infertile and will not hatch, even if incubated. A bird in the peak of health on an ideal diet may be able to sustain some egg production without serious harm. However, with captive pet birds, it can also become an obsession, because the eggs do not hatch and allow the full cycle to complete, thus turning off the hormonal trigger to lay eggs. Constant egg laying will deplete your bird of vital nutrients, and predispose her to malnutrition , osteoporosis, and life-threatening health problems, such as egg binding and yolk peritonitis. While egg laying can occur in any breed, it is most common in cockatiels, lovebirds, budgies, canaries, and finches. Egg laying can start anytime from 5 months to over 10 years of age. If you find an egg, you want to immediately correct any environmental factors that predispose your bird to lay eggs. If that does not work, your bird may require medical treatment to control egg laying, so you’ll want to get her to a qualified Avian vet. There are several safe, effective hormonal treatments available, which your Avian vet can tailor to your bird's needs. -

Nest Building and Egg Laying by Redwinged Blackbirds in Response to Artificial Manipulations
NEST BUILDING AND EGG LAYING BY REDWINGED BLACKBIRDS IN RESPONSE TO ARTIFICIAL MANIPULATIONS LARRY C. HOLCOMB THE responsesof breedingbirds to many external stimuli have been well reviewedby Lehrman (1959), Eisner (1960), and van Tienhoven (1961). The studiesperformed by Emlen (1941) on the Tricolored Blackbird (Agelaiustricolor) are especiallyrelevant to this paper because of somesimilarities of this icterid to the RedwingedBlackbird (Agelaius phoeniceus).Orians (1961) reportsthe similaritiesand differencesin the ecologyand social systemsof the Redwingedand Tricolored Blackbirds. The objectivesof the presentstudy were to assesssome of the external stimuli that affect physiologicaland related behavioral patterns in the Red-wing. The two nmin objectiveswere to 1) determineresponses of Red- wings to artificial nest manipulations,compared with thosereported by Emlen (1941) for the TricoloredBlackbird, and 2) discoverif Red-wings are determinateor indeterminatelayers. Emlen found that the TricoloredBlackbird usually lays its first egg on the day followingnest completion.Payne (1965) reportsthat Tricolored Blackbirdsusually lay their first eggin the morningfollowing completion of the nestbut that Red-wingfemales have a rangeof 1-4 days. In addition Payne notes that all Tricolored Blackbirds had ovulated on the day of lining the nest, whereassix of eight Red-wing femalestaken from lined nestshad not ovulated. I have made similar observationson Red-wings. Payne statesthat "clutchsize of RedwingedBlackbirds is apparentlydeter- mined by the developmentof the ovary a few days before the final egg is laid." Holcomb and Twiest (1968) present data on the time between nest completionand egglaying for 42 uplandand marshbreeding Red-wings in May and June. Theseshow a meandelay in laying of 2.0 days after nest completion,with a range of between0 and 5 days. -

Hatching Small Numbers of Eggs
PNW 478 Reprinted October 1995 Hatching small numbers of eggs J.C. Hermes ncubation is the process by which the embryo within the egg develops into a fully formed chick capable of breaking free from the shell. Incubat- Iing eggs requires several important environmental considerations if any chicks are to hatch. Of primary concern are temperature and relative humidity. Attention to secondary considerations can result in increased numbers of eggs hatching. Natural vs. artificial relative humidity within the proper limits to hatch chicks. incubation Artificial incubation is an The first decision to be made excellent alternative to the is whether to incubate eggs broody hen. naturally or artificially. Natural incubation uses a broody hen to Temperature incubate eggs by sitting on them Correct incubation tempera- in a nest. Broody hens, when ture depends on the type of available, work best for small incubator. In forced draft incuba- clutches of eggs. tors, those in which a fan is Broodiness, the behavior of a present, temperature should be incubators, humidity is attained setting hen, has been bred out of ° maintained between 99.5 F and by filling a pan or some other some chickens. Others don’t care ° 100 F for most bird eggs. In still reservoir in the incubator with for chicks effectively. Some air incubators, where no fan breeds commonly used to hatch water. is present, a temperature of For most eggs, a relative eggs include New Hampshires, between 101°F and 103°F Plymouth Rocks, Rhode Island humidity of between 55 and measured at the top of the egg 60 percent is adequate during the Reds, and Cochins. -

Common Birds of the Estero Bay Area
Common Birds of the Estero Bay Area Jeremy Beaulieu Lisa Andreano Michael Walgren Introduction The following is a guide to the common birds of the Estero Bay Area. Brief descriptions are provided as well as active months and status listings. Photos are primarily courtesy of Greg Smith. Species are arranged by family according to the Sibley Guide to Birds (2000). Gaviidae Red-throated Loon Gavia stellata Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: A small loon seldom seen far from salt water. In the non-breeding season they have a grey face and red throat. They have a long slender dark bill and white speckling on their dark back. Information: These birds are winter residents to the Central Coast. Wintering Red- throated Loons can gather in large numbers in Morro Bay if food is abundant. They are common on salt water of all depths but frequently forage in shallow bays and estuaries rather than far out at sea. Because their legs are located so far back, loons have difficulty walking on land and are rarely found far from water. Most loons must paddle furiously across the surface of the water before becoming airborne, but these small loons can practically spring directly into the air from land, a useful ability on its artic tundra breeding grounds. Pacific Loon Gavia pacifica Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: The Pacific Loon has a shorter neck than the Red-throated Loon. The bill is very straight and the head is very smoothly rounded. -

Titanis Walleri: Bones of Contention
Bull. Fla. Mus. Nat. Hist. (2005) 45(4): 201-229 201 TITANIS WALLERI: BONES OF CONTENTION Gina C. Gould1 and Irvy R. Quitmyer2 Titanis walleri, one of the largest and possibly the last surviving member of the otherwise South American Phorusrhacidae is re- considered in light of all available data. The only verified phorusrhacid recovered in North America, Titanis was believed to exhibit a forward-extending arm with a flexible claw instead of a traditional bird wing like the other members of this extinct group. Our review of the already described and undescribed Titanis material housed at the Florida Museum of Natural History suggest that Titanis: (1) was like other phorusrhacids in sporting small, ineffectual ratite-like wings; (2) was among the tallest of the known phorusrhacids; and (3) is the last known member of its lineage. Hypotheses of its range extending into the Pleistocene of Texas are challenged, and herein Titanis is presumed to have suffered the same fate of many other Pliocene migrants of the Great American Interchange: extinction prior to the Pleistocene. Key Words: Phorusrhacidae; Great American Biotic Interchange; Florida; Pliocene; Titanis INTRODUCTION men on the tarsometatarsus, these specimens were as- Titanis walleri (Brodkorb 1963), more commonly known signed to the Family Phorusrhacidae (Brodkorb 1963) as the North American ‘Terror Bird’, is one of the larg- and named after both a Titan Goddess from Greek my- est known phorusrhacids, an extinct group of flightless thology and Benjamin Waller, the discoverer of the fos- carnivorous birds from the Tertiary of South America, sils (Zimmer 1997). Since then, isolated Titanis mate- and most likely, the last known member of its lineage rial has been recovered from three other localities in (Brodkorb 1967; Tonni 1980; Marshall 1994; Alvarenga Florida (Table 1; Fig. -
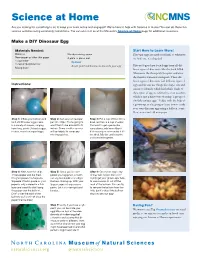
Make a DIY Dinosaur Egg (PDF)
Science at Home Are you looking for something to do to keep your brain active and engaged? We’re here to help with Science at Home! You can do these fun science activities using commonly found items. You can also visit us at the Museum’s Science at Home page for additional resources. Make a DIY Dinosaur Egg Materials Needed: Start Here to Learn More! Balloons Wooden mixing spoon Dinosaur eggs are rarely fossilized, so whenever Newspaper or other thin paper A plate or place-mat we find one, it’s a big deal. 1 cup water Optional 1 cup all-purpose flour Acrylic paint and brushes to decorate your egg Paleontologists have found eggs from all dif- Mixing bowl ferent types of dinosaurs, like the duck-billed Maiasaura, the theropod Oviraptor, and even the massive titanosaur sauropods. These dif- ferent types of dinosaurs laid different types of Instructions: eggs and we can use things like shape, size and texture to identify which laid which. Each of these types of egg are referred to as an ootaxon, which is just a fancy way of saying “a group of similarly unique eggs.” Today, with the help of a grown-up, you’re going to learn how to make your own dinosaur eggs using a balloon, some flour, water and old newspaper. Step 1: Inflate your balloon and Step 2: Cut up your newspa- Step 3: Put a cup of flour into a tie it off. Dinosaur eggs came per into strips. You’re going to bowl, and mix in a cup of water. -

The First Dinosaur Egg Remains a Mystery
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.10.406678; this version posted December 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 The first dinosaur egg remains a mystery 2 3 Lucas J. Legendre1*, David Rubilar-Rogers2, Alexander O. Vargas3, and Julia A. 4 Clarke1* 5 6 1Department of Geological Sciences, University of Texas at Austin, Austin, Texas 78756, 7 USA. 8 2Área Paleontología, Museo Nacional de Historia Natural, Casilla 787, Santiago, Chile. 9 3Departamento de Biología, Facultad de Ciencias, Universidad de Chile, Santiago 7800003, 10 Chile. 11 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.12.10.406678; this version posted December 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 12 Abstract 13 A recent study by Norell et al. (2020) described new egg specimens for two dinosaur species, 14 identified as the first soft-shelled dinosaur eggs. The authors used phylogenetic comparative 15 methods to reconstruct eggshell type in a sample of reptiles, and identified the eggs of 16 dinosaurs and archosaurs as ancestrally soft-shelled, with three independent acquisitions of a 17 hard eggshell among dinosaurs. This result contradicts previous hypotheses of hard-shelled 18 eggs as ancestral to archosaurs and dinosaurs.