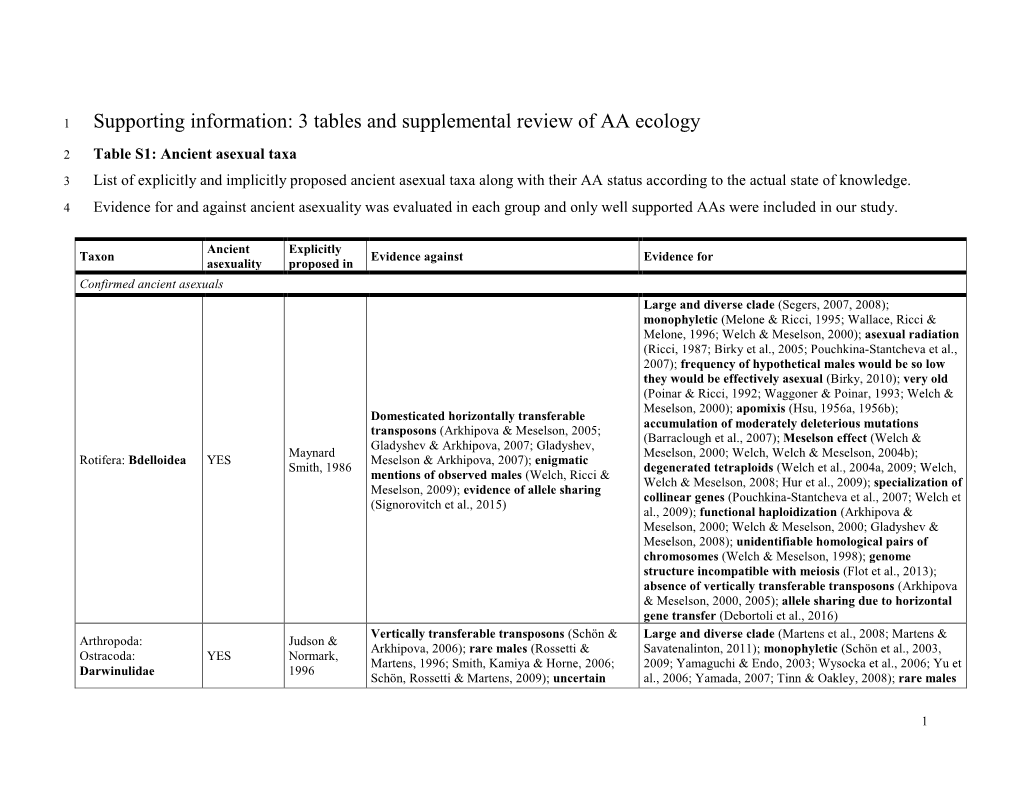
Supporting Information: 3 Tables and Supplemental Review of AA Ecology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volume 2, Chapter 10-2: Arthropods: Crustacea
Glime, J. M. 2017. Arthropods: Crustacea – Ostracoda and Amphipoda. Chapt. 10-2. In: Glime, J. M. Bryophyte Ecology. Volume 2. 10-2-1 Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 10-2 ARTHROPODS: CRUSTACEA – OSTRACODA AND AMPHPODA TABLE OF CONTENTS CLASS OSTRACODA ..................................................................................................................................... 10-2-2 Adaptations ................................................................................................................................................ 10-2-3 Swimming to Crawling ....................................................................................................................... 10-2-3 Reproduction ....................................................................................................................................... 10-2-3 Habitats ...................................................................................................................................................... 10-2-3 Terrestrial ............................................................................................................................................ 10-2-3 Peat Bogs ............................................................................................................................................ 10-2-4 Aquatic ............................................................................................................................................... -

Sex Is a Ubiquitous, Ancient, and Inherent Attribute of Eukaryotic Life
PAPER Sex is a ubiquitous, ancient, and inherent attribute of COLLOQUIUM eukaryotic life Dave Speijera,1, Julius Lukešb,c, and Marek Eliášd,1 aDepartment of Medical Biochemistry, Academic Medical Center, University of Amsterdam, 1105 AZ, Amsterdam, The Netherlands; bInstitute of Parasitology, Biology Centre, Czech Academy of Sciences, and Faculty of Sciences, University of South Bohemia, 370 05 Ceské Budejovice, Czech Republic; cCanadian Institute for Advanced Research, Toronto, ON, Canada M5G 1Z8; and dDepartment of Biology and Ecology, University of Ostrava, 710 00 Ostrava, Czech Republic Edited by John C. Avise, University of California, Irvine, CA, and approved April 8, 2015 (received for review February 14, 2015) Sexual reproduction and clonality in eukaryotes are mostly Sex in Eukaryotic Microorganisms: More Voyeurs Needed seen as exclusive, the latter being rather exceptional. This view Whereas absence of sex is considered as something scandalous for might be biased by focusing almost exclusively on metazoans. a zoologist, scientists studying protists, which represent the ma- We analyze and discuss reproduction in the context of extant jority of extant eukaryotic diversity (2), are much more ready to eukaryotic diversity, paying special attention to protists. We accept that a particular eukaryotic group has not shown any evi- present results of phylogenetically extended searches for ho- dence of sexual processes. Although sex is very well documented mologs of two proteins functioning in cell and nuclear fusion, in many protist groups, and members of some taxa, such as ciliates respectively (HAP2 and GEX1), providing indirect evidence for (Alveolata), diatoms (Stramenopiles), or green algae (Chlor- these processes in several eukaryotic lineages where sex has oplastida), even serve as models to study various aspects of sex- – not been observed yet. -

Multigene Eukaryote Phylogeny Reveals the Likely Protozoan Ancestors of Opis- Thokonts (Animals, Fungi, Choanozoans) and Amoebozoa
Accepted Manuscript Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opis- thokonts (animals, fungi, choanozoans) and Amoebozoa Thomas Cavalier-Smith, Ema E. Chao, Elizabeth A. Snell, Cédric Berney, Anna Maria Fiore-Donno, Rhodri Lewis PII: S1055-7903(14)00279-6 DOI: http://dx.doi.org/10.1016/j.ympev.2014.08.012 Reference: YMPEV 4996 To appear in: Molecular Phylogenetics and Evolution Received Date: 24 January 2014 Revised Date: 2 August 2014 Accepted Date: 11 August 2014 Please cite this article as: Cavalier-Smith, T., Chao, E.E., Snell, E.A., Berney, C., Fiore-Donno, A.M., Lewis, R., Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and Amoebozoa, Molecular Phylogenetics and Evolution (2014), doi: http://dx.doi.org/10.1016/ j.ympev.2014.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 1 Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts 2 (animals, fungi, choanozoans) and Amoebozoa 3 4 Thomas Cavalier-Smith1, Ema E. Chao1, Elizabeth A. Snell1, Cédric Berney1,2, Anna Maria 5 Fiore-Donno1,3, and Rhodri Lewis1 6 7 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -

Crustacea, Ostracoda), from Christmas Island (Indian Ocean) with Some Considerations on the Morphological Evolution of Ancient Asexuals
Belg. J. Zool., 141 (2) : 55-74 July 2011 Description of a new genus and two new species of Darwinulidae (Crustacea, Ostracoda), from Christmas Island (Indian Ocean) with some considerations on the morphological evolution of ancient asexuals Giampaolo Rossetti1*, Ricardo L. Pinto 2 & Koen Martens 3 1 University of Panna, Department of Enviromnental Sciences, Viale G.P. Usberti 33 A, 1-43100 Panna, Italy 2 University of Brasilia, Institute of Geosciences, Laboratory of Micropaleontology, ICC, Campus Universitário Darcy Ribeiro Asa Norte, 70910-900 Brasilia, DF, Brazil 3 Royal Belgian Institute of Natural Sciences, Freshwater Biology, Vautierstraat 29, B-1000 Brussels, Belgium, and University of Ghent, Department of Biology, K.L. Ledeganckstraat 35, B-9000 Gent, Belgimn * Conesponding author: Giampaolo Rossetti. Mail: giampaolo.rosscttin unipr.it ABSTRACT. Darwinulidae is believed to be one of the few metazoan taxa in which fully asexual reproduction might have persisted for millions of years. Although rare males in a single darwinulid species have recently been found, they may be non-functional atavisms. The representatives of this family are characterized by a slow evolutionary rate, resulting in a conservative morphology in the different lineages over long time frames and across wide geographic ranges. Differences between species and genera, although often based on small details of valve morphology and chaetotaxy, are nevertheless well-recognizable. Five recent genera ( Darwinula, Alicenula, Vestcdemilct, Penthesilemila and Microdarwimda) and about 35 living species, including also those left in open nomenclature, are included in this family. Previous phylogenetic analyses using both morphological characters and molecular data confirmed that the five genera are good phyletic units. -

New Species of Fossil Oribatid Mites (Acariformes, Oribatida), from the Lower Cretaceous Amber of Spain
Cretaceous Research 63 (2016) 68e76 Contents lists available at ScienceDirect Cretaceous Research journal homepage: www.elsevier.com/locate/CretRes New species of fossil oribatid mites (Acariformes, Oribatida), from the Lower Cretaceous amber of Spain * Antonio Arillo a, , Luis S. Subías a, Alba Sanchez-García b a Departamento de Zoología y Antropología Física, Facultad de Biología, Universidad Complutense, E-28040 Madrid, Spain b Departament de Dinamica de la Terra i de l'Ocea and Institut de Recerca de la Biodiversitat (IRBio), Facultat de Geologia, Universitat de Barcelona, E- 08028 Barcelona, Spain article info abstract Article history: Mites are relatively common and diverse in fossiliferous ambers, but remain essentially unstudied. Here, Received 12 November 2015 we report on five new oribatid fossil species from Lower Cretaceous Spanish amber, including repre- Received in revised form sentatives of three superfamilies, and five families of the Oribatida. Hypovertex hispanicus sp. nov. and 8 February 2016 Tenuelamellarea estefaniae sp. nov. are described from amber pieces discovered in the San Just outcrop Accepted in revised form 22 February 2016 (Teruel Province). This is the first time fossil oribatid mites have been discovered in the El Soplao outcrop Available online 3 March 2016 (Cantabria Province) and, here, we describe the following new species: Afronothrus ornosae sp. nov., Nothrus vazquezae sp. nov., and Platyliodes sellnicki sp. nov. The taxa are discussed in relation to other Keywords: Lamellareidae fossil lineages of Oribatida as well as in relation to their modern counterparts. Some of the inclusions Neoliodidae were imaged using confocal laser scanning microscopy, demonstrating the potential of this technique for Nothridae studying fossil mites in amber. -

THE FUNCTIONAL MORPHOLOGY of OTINA OTIS, a PRIMITIVE MARINE PULMONATE by J. E. Morton Department of Zoology, Queen Mary College, University of London
J. Mar. bioI.Ass. U.K. (1955) 34, II3-150 II3 Printed in Great Britain THE FUNCTIONAL MORPHOLOGY OF OTINA OTIS, A PRIMITIVE MARINE PULMONATE By J. E. Morton Department of Zoology, Queen Mary College, University of London (Text-figs. 1-12) The family Otinidae is the smallest and probably the least known among the pulmonates. Thiele (1931) places it at the base of the Basommatophora, the more primitive order of the subclass Pulmonata, in the Stirps Actophila. It contains a single species, Otina otis Turton, with a geographical range con- fined to the coasts of the British Isles and north-west France. Its northern distribution reaches as far as the Firth of Clyde, according to Jeffreys (1869), and over the greater part of its British range it appears to follow fairly closely the distribution of the barnacle Chthamalus stellatus, which here reaches its northern limit. Otina otis is a tiny snail, and its external form is limpet-like. The shell, which is well described by Jeffreys (1869), and also by Ellis (1926), measures up to 2' 5 mm in length, with a short apical visceral coil at the posterior end. It is dark chestnut brown in colour, and most resembles a minute Haliotis. A description of the ecology of Otina otis has already appeared, as portion of a study of the crevice-dwelling animals of the upper intertidal zone at Wembury (Morton, 1954). Otina is rather restricted in its mode of occurrence as regards both vertical tidal range and selection of substratum. It lives a short distance within the mouths of crevices between layers offoliaceous rocks, such as Dartmouth Slate at Wembury and Whitsands, and felsite at Kingsands, and in fissures between blocks of Staddon Grit at Rum Bay and volcanic rocks at Drake's Island. -

IV. the Oribatid Mites (Acari: Cryptostigmata)
This file was created by scanning the printed publication. Text errors identified by the software have been corrected; however, some errors may remain. United States Department of Invertebrates of the H.J. Agriculture Andrews Experimental Forest Service Pacific Northwest Forest, Western Cascade Research Station General Technical Report Mountains, Oregon: IV. PNW-217 August 1988 The Oribatid Mites (Acari: Cryptostigmata) Andrew R. Moldenke and Becky L. Fichter I ANDREW MOLDENKE and BECKY FICHTER are Research Associates, Department of Entomology, Oregon State University, Corvallis, Oregon 97331. TAXONOMIC LISTING OF PACIFIC NORTHWEST GENERA * - indicates definite records from the Pacific Northwest *Maerkelotritia 39-40, figs. 83-84 PALAEOSOMATA (=BIFEMORATINA) (=Oribotritia sensu Walker) Archeonothroidea *Mesotritia 40 *Acaronychus 32, fig. 64 *Microtritia 40-41, fig. 85 *Zachvatkinella 32, fig. 63 *Oribotritia 39, figs. 81-82 Palaeacaroidea Palaeacarus 32, fig. 61 (=Plesiotritia) *Rhysotritia 40 Ctenacaroidea *Aphelacarus 32, fig. 59 *Synichotritia 41 Beklemishevia 32, fig. 62 Perlohmannioidea *Perlohmannia 65, figs. 164-166, 188 *Ctenacarus 32, fig. 60 ENARTHRONOTA (=ARTHRONOTINA) Epilohmannioidea *Epilohmannia 65-66, figs. 167-169, Brachychthonioidea 187 *Brachychthonius 29-30, fig. 53 Eulohmannioidea *Eobrachychthonius 29 *Eulohmannia 35, figs. 67-68 *Liochthonius 29, figs. 54,55,306 DESMONOMATA Mixochthonius 29 Crotonioidea (=Nothroidea) Neobrachychthonius 29 *Camisia 36, 68. figs. 70-71, Neoliochthonius 29 73, 177-178, 308 (=Paraliochthonius) Heminothrus 71 Poecilochthonius 29 *Malaconothrus 36, fig. 74 *Sellnickochthonius 29, figs. 56-57 Mucronothrus 36 (=Brachychochthonius) Neonothrus 71 *Synchthonius 29 *Nothrus 69, fig. 179-182, Verachthonius 29 186, 310 Hypochthonioidea *Platynothrus 71, figs. 183-185 *Eniochthonius 28, figs. 51-52 309 (=Hypochthoniella) *Trhypochthonius 35, fig. 69 *Eohypochthonius 27-28, figs. 44-45 *Hypochthonius 28, figs. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

Phylogeography in Sexual and Parthenogenetic European Oribatida
GÖTTINGER ZENTRUM FÜR BIODIVERSITÄTSFORSCHUNG UND ÖKOLOGIE - GÖTTINGEN CENTRE FOR BIODIVERSITY AND ECOLOGY - Phylogeography in sexual and parthenogenetic European Oribatida Dissertation zur Erlangung des akademischen Grades eines Doctor rerum naturalium an der Georg-August Universität Göttingen vorgelegt von Dipl. Biol. Martin Julien Rosenberger aus Langen, Hessen Referent: Prof. Dr. Stefan Scheu Koreferent: PD Dr. Mark Maraun Tag der Einreichung: 21 Oktober 2010 Tag der mündlichen Prüfung: Curriculum Vitae Curriculum Vitae Personal data Name: Martin Julien Rosenberger Address: Brandenburgerstrasse 53, 63329 Egelsbach Date of Birth: October 31st 1980 Place of Birth: Langen (Hessen) Education 1987-1991 Wilhelm Leuschner Primary School, Egelsbach 1991-2000 Abitur at Dreieich-Schule, Langen 2000-2006 Study of Biology at Darmstadt University of Technology, Germany 2006-2007 Diploma thesis: “Postglaziale Kolonisation von Zentraleuropa durch parthenogenetische (Platynothrus peltifer) und sexuelle (Steganacarus magnus) Hornmilben (Oribatida)” at Darmstadt University of Technology, Germany under supervision of Dipl. Biol. Katja Domes and Prof. Dr. S. Scheu 2007-2008 Scientific assistant at Darmstadt University of Technology, Germany 2008-2009 Scientific officer Darmstadt University of Technology, Germany Since 2009 PhD student at the Georg August University, Göttingen, Germany at the J. F. Blumenbach Insitute of Zoology and Anthropology under supervision of Prof. Dr. S. Scheu 2009-2010 Scientific officer at the Georg August University, Göttingen, -

Filtering, Feeding, and Digestion in the Lamellibranch Lasaea Rubra
J. Mar. bioI.Ass. U.K. (1956) 35, 241-274 241 Printed in Great Britain FILTERING, FEEDING, AND DIGESTION IN THE LAMELLIBRANCH LASAEA RUBRA By Dorothy Ballantine The Plymouth Laboratory and J. E. Morton Department of Zoology, Queen Mary College, University of London (Text-figs. I-II) Lasaea rubra is the smallest and commonest of Plymouth bivalves. It is intertidal on rocky shores, often in immense numbers, in crevicesand other protected places, and in Pygmaeapumila (Colman, 1940).This bivalve is an excellent laboratory animal. It remains fully active for several days under laboratory conditions, and a large number of animals can be used in a single experiment, eliminating the variations in activity found when dealing with a few, or single.specimens of larger animals. Because of the relatively high position Lasaearubramay occupy on the shore its feeding cycleis broken by regular dry periods at each tide. Thus, for experiments relating to periodicity in feeding,we have an animalwhosetimes of feeding can easilybe ascertained and experimentallyvaried over a wide range. Lastly, in makingserial sections and in examiningtotal gut contents, in workon the digestivesystem,the small size of the animal is of obvious advantage. The present paper is devoted to an account of filtering rates with various food organisms, variations in filtering activity and the relation of filtering to feedingand digestion. Further workhasbeen completedby oneofus(J.E.M.) on the mechanism of digestionand the cycleof activity of the digestivegland, and experiments are in progress on respiration during submersion and exposure. We record our special thanks to Dr Mary Parke, botanist at the Plymouth Laboratory, for making availablethe unialgal cultures of the organismsused, and for her invariable kindness and sound advice. -

Hotspots of Mite New Species Discovery: Sarcoptiformes (2013–2015)
Zootaxa 4208 (2): 101–126 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Editorial ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4208.2.1 http://zoobank.org/urn:lsid:zoobank.org:pub:47690FBF-B745-4A65-8887-AADFF1189719 Hotspots of mite new species discovery: Sarcoptiformes (2013–2015) GUANG-YUN LI1 & ZHI-QIANG ZHANG1,2 1 School of Biological Sciences, the University of Auckland, Auckland, New Zealand 2 Landcare Research, 231 Morrin Road, Auckland, New Zealand; corresponding author; email: [email protected] Abstract A list of of type localities and depositories of new species of the mite order Sarciptiformes published in two journals (Zootaxa and Systematic & Applied Acarology) during 2013–2015 is presented in this paper, and trends and patterns of new species are summarised. The 242 new species are distributed unevenly among 50 families, with 62% of the total from the top 10 families. Geographically, these species are distributed unevenly among 39 countries. Most new species (72%) are from the top 10 countries, whereas 61% of the countries have only 1–3 new species each. Four of the top 10 countries are from Asia (Vietnam, China, India and The Philippines). Key words: Acari, Sarcoptiformes, new species, distribution, type locality, type depository Introduction This paper provides a list of the type localities and depositories of new species of the order Sarciptiformes (Acari: Acariformes) published in two journals (Zootaxa and Systematic & Applied Acarology (SAA)) during 2013–2015 and a summary of trends and patterns of these new species. It is a continuation of a previous paper (Liu et al. -

Ostracoda, Crustacea) in Turkey
LIMNOFISH-Journal of Limnology and Freshwater Fisheries Research 5(1): 47-59 (2019) Fossil and Recent Distribution and Ecology of Ancient Asexual Ostracod Darwinula stevensoni (Ostracoda, Crustacea) in Turkey Mehmet YAVUZATMACA * , Okan KÜLKÖYLÜOĞLU Department of Biology, Faculty of Arts and Science, Bolu Abant İzzet Baysal University, Turkey ABSTRACT ARTICLE INFO In order to determine distribution, habitat and ecological preferences of RESEARCH ARTICLE Darwinula stevensoni, data gathered from 102 samples collected in Turkey between 2000 and 2017 was evaluated. A total of 1786 individuals of D. Received : 28.08.2018 stevensoni were reported from eight different aquatic habitats in 14 provinces in Revised : 21.10.2018 six of seven geographical regions of Turkey. Although there are plenty of samples Accepted : 30.10.2018 from Central Anatolia Region, recent form of the species was not encountered. Unlike recent, fossil forms of species were encountered in all geographic regions Published : 25.04.2019 except Southeastern Anatolia. The oldest fossil record in Turkey was reported from the Miocene period (ca 23 mya). Species occurred in all climatic seasons in DOI:10.17216/LimnoFish.455722 Turkey. D. stevensoni showed high optimum and tolerance levels to different ecological variables. Results showed a positive and negative significant * CORRESPONDING AUTHOR correlations of the species with pH (P<0.05) and elevation (P<0.01), respectively. [email protected] It seems that the ecological preferences of the species are much wider than Phone : +90 537 769 46 28 previously known. Our results suggest that if D. stevensoni is used to estimate past and present environmental conditions, attention and care should be paid on its ecology and distribution.