Table S1: Parameters Used to Calculate Gas-Particle Partitioning by Absorption Theory at Three Different Values of Activity Coefficients (ℰ), Each of 0.3, 1, and 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols
life Article Evaluation of the Toxicity on Lung Cells of By-Products Present in Naphthalene Secondary Organic Aerosols Yuri Lima de Albuquerque 1 , Emmanuelle Berger 1, Sophie Tomaz 2, Christian George 2 and Alain Géloën 1,* 1 UMR Ecologie Microbienne, Université Claude Bernard Lyon 1, 69622 Villeurbanne, France; [email protected] (Y.L.d.A.); [email protected] (E.B.) 2 Univ Lyon, Université Claude Bernard Lyon 1, 69100 Villeurbanne, France; [email protected] (S.T.); [email protected] (C.G.) * Correspondence: [email protected] Abstract: In 2018, seven million people died prematurely due to exposure to pollution. Polycyclic aromatic hydrocarbons (PAHs) are a significant source of secondary organic aerosol (SOA) in urban areas. We investigated the toxic effects of by-products of naphthalene SOA on lung cells. These by- products were 1,4-naphthoquinone (1,4-NQ), 2-hydroxy-1,4-naphthoquinone (2-OH-NQ), phthalic acid (PA) and phthaldialdehyde (OPA). Two different assessment methodologies were used to monitor the toxic effects: real-time cell analysis (RTCA) and the Holomonitor, a quantitative phase contrast microscope. The chemicals were tested in concentrations of 12.5 to 100 µM for 1,4-NQ and 1 to 10 mM for 2-OH-NQ, PA and OPA. We found that 1,4-NQ is toxic to cells from 25 to 100 µM (EC50: 38.7 µM ± 5.2); 2-OH-NQ is toxic from 1 to 10mM (EC50: 5.3 mM ± 0.6); PA is toxic from 5 to 10 mM (EC50: 5.2 mM ± 0.3) and OPA is toxic from 2.5 to 10 mM (EC50: 4.2 mM ± 0.5). -

Preparative Method of Novel Phthalocyanines from 3- Nitro
Available online a t www.derpharmachemica.com Scholars Research Library Der Pharma Chemica, 2012, 4(4):1397-1403 (http://derpharmachemica.com/archive.html) ISSN 0975-413X CODEN (USA): PCHHAX Preparative Method of Novel Phthalocyanines from 3- Nitro Phthalic Anhydride, Cobalt salt and Urea with Chloromethylpolyestyrene as a Heterogenous, Reusable and Efficient Catalyst Mohammad Ali Zolfigol 2, Ali Reza Pourali 1, Sami Sajjadifar 3,4 and Shohreh Farahmand 1,2,4 1Faculty of Chemistry, Bu-Ali Sina University, Hamedan, P.O. Box 6517838683, Iran 2School of Chemistry, Damghan University, Damghan, Iran 3Department of Chemistry, Faculty of Science, Ilam University, P.O. Box 69315516, Ilam, Iran 4Department of Chemistry, Payame Noor University, Tehran, P.O. Box 19395-4697, Iran _____________________________________________________________________________________________ ABSTRACT 3-Nitrophthalic anhydride was reacted with urea and cobalt salt in nitrobenzene under N 2 at 185°C and cobalt- tetraanitrophthalocyanine (CoTNP) was produced. Cobalt-tetraaminophthalocyanine (CoTAP) was produced by reduction of CoTNP caused by Sodium borohydride under N 2(g). CoTAP and chloromethylpolystyrene was refluxed in nitrobenzene or DMF at 180 oC for 12h. The mixture was cooled down to reach the room temperature and then solvent removed and the resulting precipitate was washed with water to remove excess CoTAP, and dried it to get a light green solid (CoTAP-linked-polymer). Kaywords Phthalocyanines, Cobaltetraminophthalocyanine, CoTAP ,Phthalocyanines linked polymer. _____________________________________________________________________________________________ INTRODUCTION Phthalocyanines are of interest not only as model compounds for the biologically important porphyrins but also because the intensely colored metal complexes are of commercial importance as dyes and pigments [1], the copper derivatives being an important blue pigment [2]. -

Proposed Designation of Butyl Benzyl Phthalate As a High-Priority
United States Office of Chemical Safety and Environmental Protection Agency Pollution Prevention Proposed Designation of Butyl Benzyl Phthalate (CASRN 85-68-7) as a High-Priority Substance for Risk Evaluation August 22, 2019 Table of Contents List of Tables ................................................................................................................................ iii Acronyms and Abbreviations ..................................................................................................... iv 1. Introduction ............................................................................................................................... 1 2. Production volume or significant changes in production volume ........................................ 3 Approach ..................................................................................................................................... 3 Results and Discussion ............................................................................................................... 3 3. Conditions of use or significant changes in conditions of use ............................................... 3 Approach ..................................................................................................................................... 3 CDR Tables ................................................................................................................................. 4 CDR and TRI Summary and Additional Information on Conditions of Use ............................. 6 -

(12) United States Patent (10) Patent No.: US 8,946,374 B2 Imada Et Al
USOO894.6374B2 (12) United States Patent (10) Patent No.: US 8,946,374 B2 Imada et al. (45) Date of Patent: Feb. 3, 2015 (54) POLYETHER ESTER COMPOSITION, C08G 18/48 (2006.01) POLYURETHANE RESIN COMPOSITION, CSG 18/50 (2006.01) AND OPTICAL MATERALUSING THE (52) U.S. Cl. SAME USPC .................................. 528/79; 528/28: 528/83 (58) Field of Classification Search (75) Inventors: Tomoyuki Imada, Osaka (JP): Kouji USPC ................................................ 528/28, 79, 83 Shiraishi, Osaka (JP) See application file for complete search history. (73) Assignee: DIC Corporation, Tokyo (JP) (56) References Cited (*) Notice: Subject to any disclaimer, the term of this U.S. PATENT DOCUMENTS patent is extended or adjusted under 35 4,128,532 A * 12/1978 Eimers et al. ................... 528/79 U.S.C. 154(b) by 151 days. 5,942,158 A * 8/1999 Okoroafor et al. 252/586 2002/001643.6 A1 2/2002 Ohmori et al. .................. 528,61 (21) Appl. No.: 13/394,920 FOREIGN PATENT DOCUMENTS (22) PCT Filed: Sep. 14, 2010 JP 8-208830 A 8, 1996 JP 11-2792.51 A 10, 1999 (86). PCT No.: PCT/UP2010/065777 WO 2009 107301 A1 9, 2009 S371 (c)(1), (2), (4) Date: Mar. 8, 2012 * cited by examiner Primary Examiner — Rabon Sergent (87) PCT Pub. No.: WO2011/034033 (74) Attorney, Agent, or Firm — McDermott Will & Emery PCT Pub. Date: Mar. 24, 2011 LLP (65) Prior Publication Data (57) ABSTRACT A polyurethane resin composition for optical applications can US 2012/O172567 A1 Jul. 5, 2012 contain, as essential components, a polyisocyanate and a polyester-ether polyol obtained by polycondensation of a (30) Foreign Application Priority Data glycol component containing an alkylene oxide adduct of a bisphenol and an aromatic carboxylic acid component and Sep. -

Benzyl Butyl Phthalate Or BBP)
Toxicity Review for Benzylnbutyl Phthalate (Benzyl Butyl Phthalate or BBP) Introduction Benzyl butyl phthalate (BBP) is a man‐made phthalate ester that is mostly used in vinyl tile (CERHR, 2003). BBP can also be found as a plasticizer in polyvinyl chloride (PVC) for the manufacturing of conveyor belts, carpet, weather stripping and more. It is also found in some vinyl gloves and adhesives. BBP is produced by the sequential reaction of butanol and benzyl chloride with phthalic anhydride (CERHR, 2003). The Monsanto Company is the only US producer of BBP (IPCS, 1999). When BBP is added during the manufacturing of a product, it is not bound to the final product. However, through the use and disposal of the product, BBP can be released into the environment. BBP can be deposited on and taken up by crops for human and livestock consumption, resulting in its entry into the food chain (CERHR, 2003). Concentrations of BBP have been found in ambient and indoor air, drinking water, and soil. However, the concentrations are low and intakes from these routes are considered negligible (IPCS, 1999). Exposure to BBP in the general population is based on food intake. Occupational exposure to BBP is possible through skin contact and inhalation, but data on BBP concentrations in the occupational environment is limited. Unlike some other phthalates, BBP is not approved by the U.S. Food and Drug Administration for use in medicine or medical devices (IPCS, 1999; CERHR, 2003). Based on the National Toxicology Program (NTP) bioassay reports of increased pancreatic lesions in male rats, a tolerable daily intake of 1300 µg/kg body weight per day (µg/kg‐d) has been calculated for BBP by the International Programme on Chemical Safety (IPCS) (IPCS, 1999). -

Phthalic Anhydride for Possible Carcinogenicity
OFFICIAL NTP FILE COPY National Cancer Institute T -H I CARCINOGENESIS DO NOT REMOVE FROM THIS OFFICE Technical Report Series = NO. 159 £ 1979 I O D m BIOASSAY OF PHTHALIC ANHYDRIDE FOR POSSIBLE CARCINOGENICITY CAS No. 85-44-9 NCI-CG-TR-159 z o 6 o H 30 Ul CO U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service National Institutes of Health (O >g CO BIOASSAY OF PHTHALIC ANHYDRIDE FOR POSSIBLE CARCINOGENICITY Carcinogenesis Testing Program Division of Cancer Cause and Prevention National Cancer Institute National Institutes of Health Bethesda, Maryland 20014 U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service National Institutes of Health DHEW Publication No. (NIH) 79-1715 ii BIOASSAY OF PHTHALIC ANHYDRIDE FOR POSSIBLE CARCINOGENICITY Carcinogenesis Testing Program Division of Cancer Cause and Prevention National Cancer Institute National Institutes of Health FOREWORD; This report presents the results of the bioassay of phthalic anhydride conducted for the Carcinogenesis Testing Program, Division of Cancer Cause and Prevention, National Cancer Institute (NCI), National Institutes of Health, Bethesda, Maryland. This is one of a series of experiments designed to determine whether selected chemicals have the capacity to produce cancer in animals. Negative results, in which the test animals do not have a greater incidence of cancer than control animals, do not necessarily mean that the test chemical is not a carcino gen, inasmuch as the experiments are conducted under a limited set of circumstances. Positive results demonstrate that the test chemical is carcinogenic for animals under the conditions of the test and indicate that exposure to the chemical is a potential risk to man. -

Final Scope of the Risk Evaluation for Butyl Benzyl Phthalate CASRN 85-68-7
EPA Document# EPA-740-R-20-015 August 2020 United States Office of Chemical Safety and Environmental Protection Agency Pollution Prevention Final Scope of the Risk Evaluation for Butyl Benzyl Phthalate (1,2-Benzenedicarboxylic acid, 1-butyl 2-(phenylmethyl) ester) CASRN 85-68-7 August 2020 TABLE OF CONTENTS ACKNOWLEDGEMENTS ......................................................................................................................6 ABBREVIATIONS AND ACRONYMS ..................................................................................................7 EXECUTIVE SUMMARY .....................................................................................................................10 1 INTRODUCTION ............................................................................................................................12 2 SCOPE OF THE EVALUATION ...................................................................................................13 2.1 Reasonably Available Information ..............................................................................................13 Search of Gray Literature ...................................................................................................... 14 Search of Literature from Publicly Available Databases (Peer-reviewed Literature) ........... 15 Search of TSCA Submissions ................................................................................................ 24 2.2 Conditions of Use ........................................................................................................................25 -

Toxicological Characterization of Phthalic Acid
Toxicol. Res. Vol. 27, No. 4, pp. 191-203 (2011) Open Access http://dx.doi.org/10.5487/TR.2011.27.4.191 plSSN: 1976-8257 eISSN: 2234-2753 Toxicological Characterization of Phthalic Acid Du Yeon Bang, In Kyung Lee and Byung-Mu Lee Laboratory of Toxicology, College of Pharmacy, Sungkyunkwan University, Suwon 440-746, Korea (Received October 2, 2011; Revised October 15, 2011; Accepted October 25, 2011) There has been growing concern about the toxicity of phthalate esters. Phthalate esters are being used widely for the production of perfume, nail varnish, hairsprays and other personal/cosmetic uses. Recently, exposure to phthalates has been assessed by analyzing urine for their metabolites. The parent phthalate is rapidly metabolized to its monoester (the active metabolite) and also glucuronidated, then excreted. The objective of this study is to evaluate the toxicity of phthalic acid (PA), which is the final common meta- bolic form of phthalic acid esters (PAEs). The individual PA isomers are extensively employed in the syn- thesis of synthetic agents, for example isophthalic acid (IPA), and terephthalic acid (TPA), which have very broad applications in the preparation of phthalate ester plasticizers and components of polyester fiber, film and fabricated items. There is a broad potential for exposure by industrial workers during the manu- facturing process and by the general public (via vehicle exhausts, consumer products, etc). This review suggests that PA shows in vitro and in vivo toxicity (mutagenicity, developmental toxicity, reproductive toxicity, etc.). In addition, PA seems to be a useful biomarker for multiple exposure to PAEs in humans. Key words: Phthalic acid, Phthalates, Common metabolite, Plasticizer INTRODUCTION disrupting chemicals (EDCs). -

Quantitative and Histomorphological Studies on Age-Correlated Changes in Canine and Porcine Hypophysis Lakshminarayana Das Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1971 Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis Lakshminarayana Das Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Animal Structures Commons, and the Veterinary Anatomy Commons Recommended Citation Das, Lakshminarayana, "Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis" (1971). Retrospective Theses and Dissertations. 4873. https://lib.dr.iastate.edu/rtd/4873 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. 71-26,847 DAS, Lakshminarayana, 1936- QUANTITATIVE AND HISTOMORPHOLOGICAL STUDIES ON AGE-CORRELATED CHANGES IN CANINE AND PORCINE HYPOPHYSIS (VOLUMES I AND II). Iowa State University, Ph.D., 1971 Anatomy• University Microfilms, A XEROX Company, Ann Arbor. Michigan Quantitative and histomorphological studies on age-correlated changes in canine and porcine hypophysis py Lakshminarayana Das Volume 1 of 2 A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OP PHILOSOPHY Major Subject: -

Phthalate (DEHP) on Survival, Morphology and Skeleton System of Mice Fetuses
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2015): 78.96 | Impact Factor (2015): 6.391 Teratogenic effects of di-(2-ethylhexyl)-phthalate (DEHP) on Survival, Morphology and Skeleton System of Mice Fetuses EL-Balshy, R. M.1, Ibrahim, S.A2, Award, M.H3, El- Daly, A.A4, Iskandar, M.K.5 1, 2, 3,, 4, 5Department of Zoology- Faculty of Science- Benha University-Benha- Egypt Abstract: The present study delt with the effects of di-(2-ethylhexyl)-phthalate (DEHP) on the skeletal system of the prenatal fetuses of 20thday of gestation. DEHP treatment caused fetal mortality, retarded weight, length and ossification of some bones and shortness of others. Skull, vertebrae, sternum, girdles and limbs were the main affected by DEHP. Keywords: Skeletal system –di-(2-ethylhexyl) phthalate – Teratogenesis – Malformation. 1. Introduction Fetuses and infants who are believed to be one of the most sensitive populations to environment chemicals Di-(2-ethylhexyl)-phthalate (DEHP) is one of a variety of (Hollady&Smialowicz, 2000)can be maternally exposed to plasticizers used in the production of polyvinyl chloride DEHP. In fact, DEHP, have been detectable in human cord plastics, polyvinyl acetate, rubbers, cellulose plastics, and blood and maternal plasma (Latini et al., 2003). In addition, polyurethane resins which is one of a variety of plasticizers MEHP has been found in human breast milk (Main et al, used in the production of polyvinyl chloride plastics, 2006), and in infant (Mortensen et al., 2005 and polyvinyl acetate, rubbers, cellulose plastics, and Sorensen,2006). The previous studies have suggested that polyurethane resins(Hauser &Calafat 2005). -

Tables Table S1. Concentrations of Measured Particulate Organic
Tables Table S1. Concentrations of measured particulate organic species in PM2.5 at PKU and Yufa site in summer of Beijing 2008 (ng/m3) PKU Yufa Compound average std average std Non-polar compounds Naphthalene1 2.4 0.8 0.9 0.4 Acenaphthylene1 0.3 0.2 0.3 0.4 Acenapthene1 0.3 0.2 0.2 0.2 Fluorene 0.5 0.3 0.4 0.3 1-Methylnaphthalene1 1.4 0.5 0.9 0.6 2-Methylnaphthalene1 0.9 0.4 0.8 0.6 2,6-Dimethylnaphthalene1 0.4 0.2 0.2 0.3 Methyl-fluorene 0.7 0.5 0.8 0.5 Dibenzofuran 0.0 0.0 0.0 0.0 Phenanthrene 0.9 0.7 0.8 0.4 Anthracene 0.2 0.1 0.2 0.2 Fluoranthene 1.1 0.6 1.1 0.4 Pyrene 1.0 0.5 1.1 0.5 Retene 0.5 0.3 0.6 0.3 Methyl-fluoranthene 0.1 0.1 0.1 0.1 9-Methylanthracene 0.0 0.1 0.1 0.1 Benzo[a]anthracene 0.8 0.9 1.4 0.9 Chrysene 1.3 0.8 1.4 1.0 Benzo[b]fluoranthene* 2.3 1.5 2.1 1.3 Benzo[k]fluoranthene* 0.7 0.5 1.4 1.0 Benzo[e]pyrene* 1.3 0.9 1.3 0.9 Benzo[a]pyrene 1.1 0.8 0.6 0.7 PKU Yufa Compound average std average std Benzo[ghi]flouranthene 0.6 0.4 0.6 0.3 Cyclopenta[cd]pyrene 0.6 0.5 0.9 0.8 Methyl-chrysene 0.1 0.1 0.1 0.1 Indeno[1,2,3-cd]pyrene* 1.2 0.9 1.3 1.2 Benzo[ghi]perylene 2.6 7.1 2.9 2.5 Dibenzo[a,h]anthracene* 0.1 0.2 0.2 0.4 Picene* 0.1 0.3 0.2 0.5 Coronene 0.3 0.4 0.1 0.2 Perylene 0.2 0.3 0.3 0.2 Dodecane1 1.2 0.8 1.9 2.2 Tridecane1 1.9 0.8 2.3 1.2 Tetradecane1 2.7 1.1 3.0 1.4 Pentadecane1 4.7 2.2 5.0 2.1 Hexadecane1 6.6 3.2 7.8 3.9 Norpristane1 4.5 2.6 4.6 2.6 Heptadecanoic1 9.3 5.4 11.9 6.9 Pristane1 1.8 1.1 1.8 1.0 Octadecane1 3.5 2.0 4.1 1.5 Phytane1 2.3 1.2 2.5 0.9 Nonadecane1 3.9 2.2 5.2 1.6 2-Methylnonadecane1 1.9 -

Evaluation of Anthropogenic Secondary Organic Aerosol Tracers from Aromatic Hydrocarbons
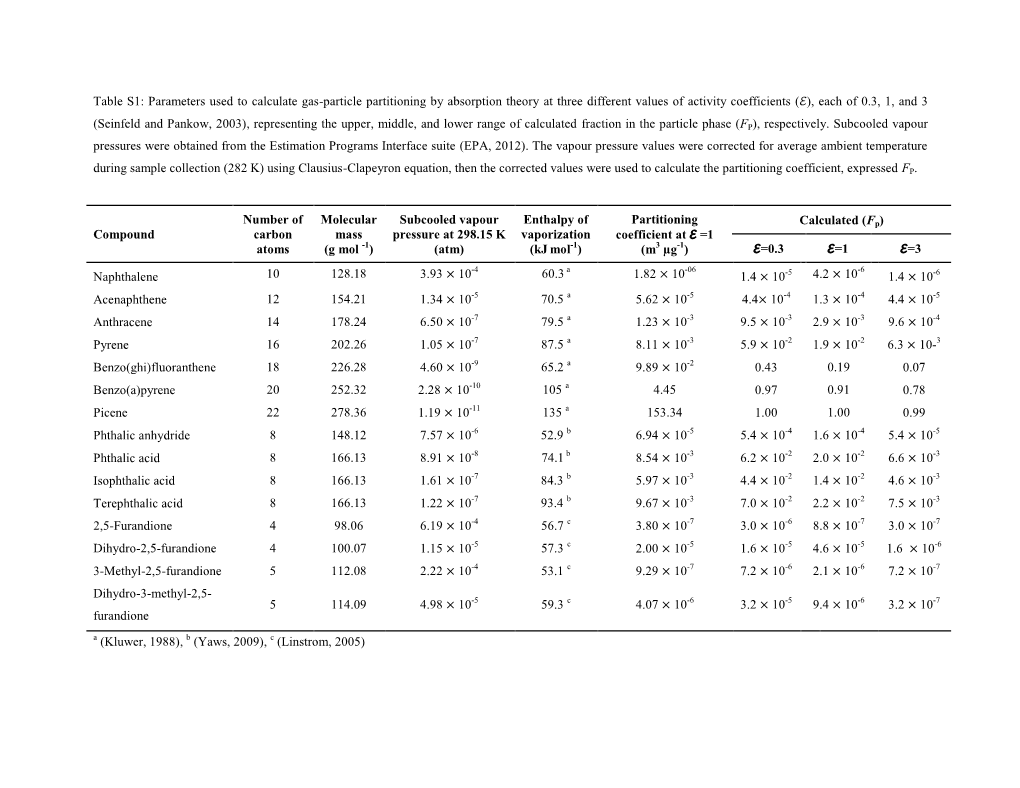
Atmos. Chem. Phys., 17, 2053–2065, 2017 www.atmos-chem-phys.net/17/2053/2017/ doi:10.5194/acp-17-2053-2017 © Author(s) 2017. CC Attribution 3.0 License. Evaluation of anthropogenic secondary organic aerosol tracers from aromatic hydrocarbons Ibrahim M. Al-Naiema and Elizabeth A. Stone Department of Chemistry, University of Iowa, Iowa City, IA 52242, USA Correspondence to: Elizabeth A. Stone ([email protected]) Received: 8 September 2016 – Discussion started: 13 September 2016 Revised: 21 December 2016 – Accepted: 7 January 2017 – Published: 10 February 2017 Abstract. Products of secondary organic aerosol (SOA) attributed to the photooxidation of aromatic hydrocarbons; from aromatic volatile organic compounds (VOCs) – however the substantial partitioning toward the gas phase 2,3-dihydroxy-4-oxopentanoic acid, dicarboxylic acids, ni- (Fp ≤ 0.16) and their water sensitivity limit their applica- tromonoaromatics, and furandiones – were evaluated for tion as tracers. The outcome of this study is the demonstra- their potential to serve as anthropogenic SOA tracers with tion that 2,3-dihydroxy-4-oxopentanoic acid, phthalic acid, respect to their (1) ambient concentrations and detectabil- 4-methylphthalic acid, and 4-hydroxy-3-nitrobenzyl alcohol ity in PM2:5 in Iowa City, IA, USA; (2) gas–particle par- are good candidates for tracing SOA from aromatic VOCs. titioning behaviour; and (3) source specificity by way of correlations with primary and secondary source tracers and literature review. A widely used tracer for toluene-derived SOA, 2,3-dihydroxy-4-oxopentanoic acid was only detected 1 Introduction in the particle phase (Fp D 1) at low but consistently mea- surable ambient concentrations (averaging 0.3 ng m−3/.