Changes in Bird Assemblages Because of Vegetation Homogenisation in Communal Livestock Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patterns of Bird Diversity in Kruger National Park, South Africa: Insights from Distribution Modelling Using Point Count Data in 2009
Patterns of bird diversity in Kruger National Park, South Africa: insights from distribution modelling using point count data in 2009 Peter Long1,2 & Frazer Higgins1,2 1 Department of Biology and Biochemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK +44 (0)1225 385437 [email protected] 2 Operation Wallacea Ltd. Hope House, Old Bolingbroke, Spilsby, Lincolnshire, PE23 4EX, UK Introduction Kruger National Park in South Africa covers an area of 18,989 square kilometres, making it one of the largest game reserves in Africa. It has become a major tourist attraction, largely due to the biodiversity within the park which comprises Baobab sandveld, Mopane scrub, Lebombo knobthorn-marula bushveld, mixed acacia thicket, Combretum-silver clusterleaf woodland and riverine forest ecosystems. Kruger supports over 500 species of birds, almost 150 species of mammal (including all of the Big Five), over 100 species of reptile and almost 2000 plant species. Operation Wallacea and WEI have worked in partnership with SANparks to collect biodiversity monitoring data in Kruger. Between 4th July and 12th August 2009, teams of volunteers led by experienced guides conducted bird point counts across Kruger National Park, South Africa. Additionally point counts took place in Nzikazi concession of Kruger from 28th April until 12th August. The aim of this study is to use this dataset to explore patterns of bird diversity in the park and develop recommendations for monitoring birds in Kruger. In this study we use a high-quality field monitoring dataset for an assemblage of birds integrated in GIS with readily available environmental data derived from remote sensing in order to model and validate the distributions of 51 bird species in Kruger. -

Multilocus Phylogeny of the Avian Family Alaudidae (Larks) Reveals
1 Multilocus phylogeny of the avian family Alaudidae (larks) 2 reveals complex morphological evolution, non- 3 monophyletic genera and hidden species diversity 4 5 Per Alströma,b,c*, Keith N. Barnesc, Urban Olssond, F. Keith Barkere, Paulette Bloomerf, 6 Aleem Ahmed Khang, Masood Ahmed Qureshig, Alban Guillaumeth, Pierre-André Crocheti, 7 Peter G. Ryanc 8 9 a Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese 10 Academy of Sciences, Chaoyang District, Beijing, 100101, P. R. China 11 b Swedish Species Information Centre, Swedish University of Agricultural Sciences, Box 7007, 12 SE-750 07 Uppsala, Sweden 13 c Percy FitzPatrick Institute of African Ornithology, DST/NRF Centre of Excellence, 14 University of Cape Town, Rondebosch 7700, South Africa 15 d Systematics and Biodiversity, Gothenburg University, Department of Zoology, Box 463, SE- 16 405 30 Göteborg, Sweden 17 e Bell Museum of Natural History and Department of Ecology, Evolution and Behavior, 18 University of Minnesota, 1987 Upper Buford Circle, St. Paul, MN 55108, USA 19 f Percy FitzPatrick Institute Centre of Excellence, Department of Genetics, University of 20 Pretoria, Hatfield, 0083, South Africa 21 g Institute of Pure & Applied Biology, Bahauddin Zakariya University, 60800, Multan, 22 Pakistan 23 h Department of Biology, Trent University, DNA Building, Peterborough, ON K9J 7B8, 24 Canada 25 i CEFE/CNRS Campus du CNRS 1919, route de Mende, 34293 Montpellier, France 26 27 * Corresponding author: Key Laboratory of Zoological Systematics and Evolution, Institute of 28 Zoology, Chinese Academy of Sciences, Chaoyang District, Beijing, 100101, P. R. China; E- 29 mail: [email protected] 30 1 31 ABSTRACT 32 The Alaudidae (larks) is a large family of songbirds in the superfamily Sylvioidea. -

Protected Area Management Plan Development - SAPO NATIONAL PARK
Technical Assistance Report Protected Area Management Plan Development - SAPO NATIONAL PARK - Sapo National Park -Vision Statement By the year 2010, a fully restored biodiversity, and well-maintained, properly managed Sapo National Park, with increased public understanding and acceptance, and improved quality of life in communities surrounding the Park. A Cooperative Accomplishment of USDA Forest Service, Forestry Development Authority and Conservation International Steve Anderson and Dennis Gordon- USDA Forest Service May 29, 2005 to June 17, 2005 - 1 - USDA Forest Service, Forestry Development Authority and Conservation International Protected Area Development Management Plan Development Technical Assistance Report Steve Anderson and Dennis Gordon 17 June 2005 Goal Provide support to the FDA, CI and FFI to review and update the Sapo NP management plan, establish a management plan template, develop a program of activities for implementing the plan, and train FDA staff in developing future management plans. Summary Week 1 – Arrived in Monrovia on 29 May and met with Forestry Development Authority (FDA) staff and our two counterpart hosts, Theo Freeman and Morris Kamara, heads of the Wildlife Conservation and Protected Area Management and Protected Area Management respectively. We decided to concentrate on the immediate implementation needs for Sapo NP rather than a revision of existing management plan. The four of us, along with Tyler Christie of Conservation International (CI), worked in the CI office on the following topics: FDA Immediate -

Important Bird and Biodiversity Areas of South Africa
IMPORTANT BIRD AND BIODIVERSITY AREAS of South Africa INTRODUCTION 101 Recommended citation: Marnewick MD, Retief EF, Theron NT, Wright DR, Anderson TA. 2015. Important Bird and Biodiversity Areas of South Africa. Johannesburg: BirdLife South Africa. First published 1998 Second edition 2015 BirdLife South Africa’s Important Bird and Biodiversity Areas Programme acknowledges the huge contribution that the first IBA directory (1998) made to this revision of the South African IBA network. The editor and co-author Keith Barnes and the co-authors of the various chapters – David Johnson, Rick Nuttall, Warwick Tarboton, Barry Taylor, Brian Colahan and Mark Anderson – are acknowledged for their work in laying the foundation for this revision. The Animal Demography Unit is also acknowledged for championing the publication of the monumental first edition. Copyright: © 2015 BirdLife South Africa The intellectual property rights of this publication belong to BirdLife South Africa. All rights reserved. BirdLife South Africa is a registered non-profit, non-governmental organisation (NGO) that works to conserve wild birds, their habitats and wider biodiversity in South Africa, through research, monitoring, lobbying, conservation and awareness-raising actions. It was formed in 1996 when the IMPORTANT South African Ornithological Society became a country partner of BirdLife International. BirdLife South Africa is the national Partner of BirdLife BIRD AND International, a global Partnership of nature conservation organisations working in more than 100 countries worldwide. BirdLife South Africa, Private Bag X5000, Parklands, 2121, South Africa BIODIVERSITY Website: www.birdlife.org.za • E-mail: [email protected] Tel.: +27 11 789 1122 • Fax: +27 11 789 5188 AREAS Publisher: BirdLife South Africa Texts: Daniel Marnewick, Ernst Retief, Nicholas Theron, Dale Wright and Tania Anderson of South Africa Mapping: Ernst Retief and Bryony van Wyk Copy editing: Leni Martin Design: Bryony van Wyk Print management: Loveprint (Pty) Ltd Mitsui & Co. -

Multilocus Phylogeny of the Avian Family Alaudidae (Larks) Reveals Complex Morphological Evolution, Non-Monophyletic Genera and Hidden Species Diversity
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/241692441 Multilocus phylogeny of the avian family Alaudidae (larks) reveals complex morphological evolution, non-monophyletic... Article in Molecular Phylogenetics and Evolution · June 2013 DOI: 10.1016/j.ympev.2013.06.005 · Source: PubMed CITATIONS READS 15 430 10 authors, including: Urban Olsson Frederick Keith Barker University of Gothenburg University of Minnesota Twin Cities 152 PUBLICATIONS 1,813 CITATIONS 181 PUBLICATIONS 3,373 CITATIONS SEE PROFILE SEE PROFILE Aleem Ahmed Khan Peter G Ryan Bahauddin Zakariya University Percy FitzPatrick Institute of African Ornithol… 67 PUBLICATIONS 1,249 CITATIONS 431 PUBLICATIONS 8,138 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Foraging ecology of African Penguins and Cape Cormorants at Stony Point and Dyer Island - developing tools to inform marine ecosystem management View project PATHOCENE: Prehistoric pathogen research initiative: Microscopic and genomic analysis of disease vectors and pathogens from southern African Pleistocene archaeological contexts View project All content following this page was uploaded by Aleem Ahmed Khan on 30 August 2017. The user has requested enhancement of the downloaded file. YMPEV 4625 No. of Pages 14, Model 5G 25 June 2013 Molecular Phylogenetics and Evolution xxx (2013) xxx–xxx 1 Contents lists available at SciVerse ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev 6 7 3 Multilocus phylogeny of the avian family Alaudidae (larks) reveals 4 complex morphological evolution, non-monophyletic genera and hidden 5 species diversity a,b,c,⇑ c d e f 8 Q1 Per Alström , Keith N. -

The Birds of Africa, Comprising All the Species Which Occur in The
: ^rpl, THE BIRDS OF AFRICA, COMPRISING ALL THE SPECIES WHICH OCCUR ETHIOPIAN REGION. BY &C. G. E. SHELLEY, F.Z.S., F.R.G.S., (late gkenadier guaeds), aitthor of "a handbook to the birds of egypt,' "a monograph of the sunbirds," etc. VOL. III. LONDON PUBLISHED FOR THE AUTHOR EY CAVENDISH SQUARE, W. E. H. POETER, 7, PEINCES STEEET, 1902. liw^<J^ ? SEP 18 1902/ ^fiiii CONTENTS. vi. LIST OF PLATES—VOL. IIL Plate XV., Ordei- I. PASSERIFORMES. Suborder II. OSCINES. Section II. ALAUD^. Family VII. MOTACILLID.^. Genus III. MACRONYX. The Long-clawR, as Dr. Bovvdler Sharpe calls them, in the " Birds of South Africa," may be described as heavily-built Pipits. Their feet are extremely large, the hind claw long, and also the tarsus, so that the outstretched feet extend well beyond the end of the tail, although the tail is not abnormally short. This character, together with the bright colouring of the throat, and often of the breast, render the species of this genus easily recognisable. Anatomically they are Pipits. Type. iMacronyx, Swains. Zool. .Journ, iii. p. .344 (1817) .... M. capensis. KEY TO THE SPECIES. n. Five outer pairs of tail-feathers with white ends ; throat and centre of breast reddish orange capensis. 2. h. Four outer pairs of tail-feathers with white ends ; no shade of red on the throat or breast. «^. Throat and some of the breast bright lemon yellow. «2. Smaller ; wing less than 4 inches ; upper croceus. parts paler ; less brown on the breast V b^. Larger; wing 4-2 to 4-4; upper parts darker ; more brown on the breast . -

Appendix F Specialist Studies
APPENDIX F SPECIALIST STUDIES The following Specialist Studies have been conducted for the compilation of the Draft Environmental impact Assessment Report. F1. Fisheries Study F2. Marine Ecology F3. Terrestrial Ecology F4. Maritime Heritage APPENDIX F1 FISHERIES STUDY ENVIRONMENTAL IMPACT ASSESSMENT (EIA) FOR THE PROPOSED METISS SUBSEA CABLE SYSTEM OFF THE EAST COAST OF SOUTH AFRICA FISHERIES STUDY Date: March 2019 Prepared for: Environmental Resources Management South Africa (Pty) Ltd On behalf of the applicant: Prepared by: Capricorn Marine Environmental (Pty) Ltd Contact Details: Sarah Wilkinson P.O. Box 50035, Waterfront Cape Town, 8002 South Africa T : +27 21 4252161 F : +27 21 4251994 www.capfish.co.za 26 March 2019 This report was prepared by Sarah Wilkinson and David Japp of CapMarine (Pty) Ltd. David Japp has a BSC in Zoology, University of Cape Town (UCT) and an MSc degree in Fisheries Science from Rhodes University. Sarah Wilkinson has a BSc (Hons) degree in Botany from UCT. Both are professional natural scientists registered with the SA Council for Natural Scientific Professions (SACNASP). Mr Japp has worked in the field of Fisheries Science and resource assessment since 1987 and has considerable experience in undertaking specialist environmental impact assessments relating to fishing and fish stocks. His work has included environmental economic assessments and the evaluation of the environmental impacts on fishing. Sarah Wilkinson has worked on marine resource assessments, specializing in spatial and temporal analysis (GIS) as well as the economic impacts of fisheries exploitation in the southern African region. This specialist report was compiled for Environmental Resources Management (ERM) for their use in compiling a Scoping Report and Environmental Impact Assessment (EIA) for the proposed METISS Submarine Cable System off the East Coast of South Africa. -

Birds, Reptiles, Fish, Insects, Aquatic Invertebrates and Ecosystems
AWF FOUR CORNERS TBNRM PROJECT : REVIEWS OF EXISTING BIODIVERSITY INFORMATION i Published for The African Wildlife Foundation's FOUR CORNERS TBNRM PROJECT by THE ZAMBEZI SOCIETY and THE BIODIVERSITY FOUNDATION FOR AFRICA 2004 PARTNERS IN BIODIVERSITY The Zambezi Society The Biodiversity Foundation for Africa P O Box HG774 P O Box FM730 Highlands Famona Harare Bulawayo Zimbabwe Zimbabwe Tel: +263 4 747002-5 E-mail: [email protected] E-mail: [email protected] Website: www.biodiversityfoundation.org Website : www.zamsoc.org The Zambezi Society and The Biodiversity Foundation for Africa are working as partners within the African Wildlife Foundation's Four Corners TBNRM project. The Biodiversity Foundation for Africa is responsible for acquiring technical information on the biodiversity of the project area. The Zambezi Society will be interpreting this information into user-friendly formats for stakeholders in the Four Corners area, and then disseminating it to these stakeholders. THE BIODIVERSITY FOUNDATION FOR AFRICA (BFA is a non-profit making Trust, formed in Bulawayo in 1992 by a group of concerned scientists and environmentalists. Individual BFA members have expertise in biological groups including plants, vegetation, mammals, birds, reptiles, fish, insects, aquatic invertebrates and ecosystems. The major objective of the BFA is to undertake biological research into the biodiversity of sub-Saharan Africa, and to make the resulting information more accessible. Towards this end it provides technical, ecological and biosystematic expertise. THE ZAMBEZI SOCIETY was established in 1982. Its goals include the conservation of biological diversity and wilderness in the Zambezi Basin through the application of sustainable, scientifically sound natural resource management strategies. -

GHANA: Picathartes and Plover a Tropical Birding Set Departure
GHANA: Picathartes and Plover A Tropical Birding Set Departure April 3 – 18, 2010 Guide: Ken Behrens All photos by Ken Behrens. All except one taken during this trip. INTRODUCTION Although Ghana is a relatively unknown destination for birding tours, it is quickly becoming the premier destination in West Africa. In contrast to most of the other countries in the region, it is safe, and the people are remarkably friendly. Its infrastructure is also exceptional, with good hotels, restaurants, and roads through most of the country. In terms of birding, Ghana has a huge amount to offer. The southern rainforests are a great place to find Upper Guinea endemics (restricted to the rainforest west of Togo) as well as a full range of West African forest species. Meanwhile, the northern portion of Ghana lies in the Guinea savanna belt, with a completely different set of birds. The most exceptional thing about Ghana from a birding perspective is the list of 'marquee species' that it holds. Foremost among these is Yellow-headed Picathartes, one of the most spectacular and sought-after birds in the world. Ghana is the best place to see this species, and sightings here are virtually guaranteed, whereas stories of missing Cameroon's elusive Red-headed Picathartes, abound. Put simply, Ghana is the best place in the world to see a Picathartes. In addition, Egyptian Plover has become a regular feature on this tour, after the confirmation of its presence at a new site during scouting prior to last yearʼs tour. Other spectacular birds likely in Ghana include Hartlaubʼs Duck, Congo Serpent-Eagle, Long-tailed Hawk, White-throated and Forest Francolins, Great Blue, Yellow-billed and Violet Turacos, Fraser's Eagle-Owl, Standard-winged Nightjar, Shining-blue, Dwarf, and Blue-breasted Kingfishers, Blue-headed, Red-throated, and Rosy Bee-eaters, White-crested, Black Dwarf, Brown-cheeked, Black-casqued, and Yellow-casqued Hornbills, Fire-bellied Woodpecker, Rufous-sided Broadbill, Blue Cuckoo-shrike, Green-headed, Buff-throated, and Reichenbachʼs Sunbirds, and Long-tailed Glossy-Starling. -

Namibian Bird List (Version 2021-01-01, Edited by Holger Kolberg) This List Is Based On: Brown, C.J., J.M
Namibian Bird List (Version 2021-01-01, edited by Holger Kolberg) This list is based on: Brown, C.J., J.M. Mendelsohn, N. Thomson and M. Boorman 2017. Checklist and analysis of the birds of Namibia as at 31 January 2016. Biological Observations 8.20: 1-153. Published online: 22 April 2017. (http://bo.adu.org.za/content.php?id=315) and subsequent updates. The scientific names in this list were edited using the latest versions of three global checklists viz.: Birds of the World (BOW): The Cornell Lab of Ornithology Birds of the World https://birdsoftheworld.org/bow/home Clements v2019 (Clements): Clements, J. F., T. S. Schulenberg, M. J. Iliff, S. M. Billerman, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2019. The eBird/Clements Checklist of Birds of the World: v2019. https://www.birds.cornell.edu/clementschecklist/download/ IOC World Bird List 10.2 (IOC): Gill, F., D. Donsker and P. Rasmussen (Eds). 2020. IOC World Bird List (v 10.2). Doi 10.14344/IOC.ML.10.2. http://www.worldbirdnames.org/ Majority rule was used i.e. if two or more lists agreed on a scientific name it was used. Following Brown et al (2017) abbreviated codes are provided for the status of each species and subspecies on the list as follows: RB – Resident breeder, MB – Migrant breeder, NB – Naturalized breeder, FB – Former breeder, NM - Non-breeding migrant, PM – Passage migrant (occurs at least annually on passage), SM – Scarce migrant (occurs less than annually, but there have been more than 20 records), V – Vagrant (there are 20 or fewer Namibian records); (IA) – -
Birds of the Luangwa Valley Jul 2013
Birds of the Luangwa Valley Jul 2013 ENGLISH NAME SCIENTIFIC NAME STATUS SLNP NLNP Luambe XP Little Grebe Tachybaptus ruficollis PP 1 1 White-breasted Cormorant Phalacrocorax carbo PP 1 1 Reed Cormorant Phalacrocorax africanus PP 1 1 African Darter Anhinga rufa PP 1 1 1 White Pelican Pelecanus onocrotalus PP 1 1 1 Pink-backed Pelican Pelecanus rufescens PP 1 1 Little Bittern Ixobrychus minutus P-min PP-pa 1 1 Dwarf Bittern Ixobrychus sturmii A 1 1 Black-crowned Night Heron Nycticorax nycticorax PP 1 1 White-backed Night Heron Gorsachius leuconotus R 1 1 1 Common Squacco Heron Ardeola ralloides PP 1 1 1 Madagascar Squacco Heron Ardeola idae A-RR-CC 1 1 Rufous-bellied Heron Ardeola rufiventris PP 1 1 Cattle Egret Bubulcus ibis A 1 1 1 Green-backed Heron Butorides striata R 1 1 1 Black Egret Egretta ardesiaca PP 1 1 1 Little Egret Egretta garzetta PP 1 1 1 Western Reef Heron Egretta gularis PP-RR 1 Yellow-billed Egret Egretta intermedia PP 1 1 Great White Egret Egretta alba PP 1 1 1 Purple Heron Ardea purpurea R 1 1 Grey Heron Ardea cinerea PP 1 1 1 Black-headed Heron Ardea melanocephala PP 1 1 1 Goliath Heron Ardea goliath R-cc 1 1 1 Hamerkop Scopus umbretta R 1 1 1 Yellow-billed Stork Mycteria ibis PP 1 1 1 Openbill Stork Anastomus lamelligerus PP 1 1 1 Black Stork Ciconia nigra PP 1 1 Abdim’s Stork Ciconia abdimii A 1 1 1 Woolly-necked Stork Ciconia episcopus PP 1 1 1 White Stork Ciconia ciconia P 1 1 1 Saddle-billed Stork Ephippiorhynchus senegalensis R-cc 1 1 1 Marabou Stork Leptoptilos crumeniferus PP 1 1 1 Sacred Ibis Threskiornis -
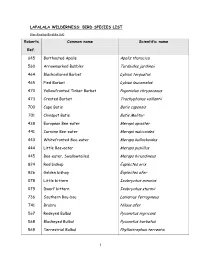
Lapalala Wilderness: Bird Species List
LAPALALA WILDERNESS: BIRD SPECIES LIST (Verified by Birdlife SA) Roberts Common name Scientific name Ref. 645 Barthoated Apalis Apalis thoracica 560 Arrowmarked Babbler Turdoides jardineii 464 Blackcollared Barbet Lybius torquatus 465 Pied Barbet Lybius leucomelas 470 Yellowfronted Tinker Barbet Pogoniolus chrysoconus 473 Crested Barbet Trachyphonus vaillantii 700 Cape Batis Batis capensis 701 Chinspot Batis Batis Molitor 438 European Bee-eater Merops apiaster 441 Carmine Bee-eater Merops nubicoides 443 Whitefronted Bee-eater Merops bullockoides 444 Little Bee-eater Merops pusiillus 445 Bee-eater, Swallowtailed Merops hirundineus 824 Red bishop Euplectes orix 826 Golden bishop Euplectes afer 078 Little bittern Ixobrychus minutus 079 Dwarf bittern Ixobrychus sturmii 736 Southern Bou-bou Laniarius ferrugineus 741 Brubru Nilaus afer 567 Redeyed Bulbul Pyconotus nigricans 568 Blackeyed Bulbul Pyconotus barbatus 569 Terrestrial Bulbul Phyllastrephus terrestis 1 574 Yellowbellied Bulbul Chlorocichla flaviventris 884 Goldenbreasted Bunting Emberiza tahapisi 886 Rock Bunting Emberiza flaviventris 887 Larklike Bunting Emberiza impetuani 230 Kori Bustard Ardeotis kori 231 Stanley’s Bustard Neotis denhami 205 Kurrichane Buttonquail Turnix sylvatica 130 Honey Buzzard Pernis apivorus 149 Steppe Buzzard Buteo buteo 152 Jackal Buzzard Buteo rufofuscus 154 Lizard Buzzard Kaupifalco monogrammicus 869 Yelloweyed canary Serinus mozambicus 870 Blackthroated canary Serinus atrogularis 881 Streakyheaded canary Serinus gularis 589 Familiar Chat Cercomela familiaris