Sodium Lauryl Sulfate Crops 1 2 Identification of Petitioned Substance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Dodecyl Sulfate
Catalog Number: 102918, 190522, 194831, 198957, 811030, 811032, 811033, 811034, 811036 Sodium dodecyl sulfate Structure: Molecular Formula: C12H25NaSO4 Molecular Weight: 288.38 CAS #: 151-21-3 Synonyms: SDS; Lauryl sulfate sodium salt; Dodecyl sulfate sodium salt; Dodecyl sodium sulfate; Sodium lauryl sulfate; Sulfuric acid monododecyl ester sodium salt Physical Appearance: White granular powder Critical Micelle Concentration (CMC): 8.27 mM (Detergents with high CMC values are generally easy to remove by dilution; detergents with low CMC values are advantageous for separations on the basis of molecular weight. As a general rule, detergents should be used at their CMC and at a detergent-to-protein weight ratio of approximately ten. 13,14 Aggregation Number: 62 Solubility: Soluble in water (200 mg/ml - clear, faint yellow solution), and ethanol (0.1g/10 ml) Description: An anionic detergent3 typically used to solubilize8 and denature proteins for electrophoresis.4,5 SDS has also been used in large-scale phenol extraction of RNA to promote the dissociation of protein from nucleic acids when extracting from biological material.12 Most proteins bind SDS in a ratio of 1.4 grams SDS to 1 gram protein. The charges intrinsic to the protein become insignificant compared to the overall negative charge provided by the bound SDS. The charge to mass ratio is essentially the same for each protein and will migrate in the gel based only on protein size. Typical Working Concentration: > 10 mg SDS/mg protein Typical Buffer Compositions: SDS Electrophoresis -

Sodium Dodecyl Sulfate-Coated Alumina and C18 Cartridge for The
J. Braz. Chem. Soc., Vol. 19, No. 8, 1523-1530, 2008. Printed in Brazil - ©2008 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00 Article Sodium Dodecyl Sulfate-Coated Alumina and C18 Cartridge for the Separation and Preconcentration of Cationic Surfactants Prior to their Quantitation by Spectrophotometric Method Mohammad Ali Karimi,*,a,b Reza Behjatmanesh-Ardakani,b Ali Aghaei Goudi b and Sara Zali b aDepartment of Chemistry, Faculty of Science, Payame Noor University (PNU), Sirjan, Iran bDepartment of Chemistry, Faculty of Science, Payame Noor University (PNU), Ardakan, Iran Um novo método de extração em fase sólida foi desenvolvido para separar e pré-concentrar traços de tensoativos catiônicos, tais como, brometo de dodeciltrimetilamônio (DTAB), brometo de cetiltrimetilamônio (CTAB) e cloreto de cetilpiridínio (CPC). Esse método é baseado na sorção do tensoativo aniônico (AS−), dodecilssulfato de sódio (SDS), sobre a superfície de γ-alumina, + enquanto um cartucho C18 é utilizado para a pré-concentração dos tensoativos catiônicos (CS ). O método espectrofotométrico, utilizado para a determinação dos tensoativos catiônicos, baseia-se na competição entre o corante catiônico, azul de metileno (MB+), e o CS+, para associação e formação do complexo SDS. O íon complexo formado (MB+) pode ser quantitativamente substituído pelo CS+, levando a um aumento da absorvância medida em 662 nm. Foram estabelecidas ótimas condições experimentais para a separação, pré-concentração e determinação dos tensoativos catiônicos. Sob essas condições otimizadas, realizou-se a pré-concentração (2×) e os resultados mostraram que a determinação do CPC, DTAB e CTAB poderia ser realizada nas faixas de concentração de 1×10-5-2×10-4, 4×10-5-5×10-4 and 5×10-5-5×10-4 mol L-1, respectivamente. -

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

Sodium Lauryl Sulfate 1 Sodium Lauryl Sulfate
Sodium lauryl sulfate 1 Sodium lauryl sulfate Sodium dodecyl sulfate Identifiers [1] CAS number 151-21-3 [2] ATC code A06 AG11 Properties Molecular formula NaC H SO 12 25 4 Molar mass 288.38 g mol−1 Density 1.01 g/cm³ Melting point 206 °C [3] (what is this?) (verify) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Sodium lauryl sulfate (SLS), sodium laurilsulfate or sodium dodecyl sulfate (SDS or NaDS) (C H SO Na) is 12 25 4 an anionic surfactant used in many cleaning and hygiene products. The molecule has a tail of 12 carbon atoms, attached to a sulfate group, giving the molecule the amphiphilic properties required of a detergent. SLS is a highly effective surfactant and is used in any task requiring the removal of oily stains and residues. For example, it is found in higher concentrations with industrial products including engine degreasers, floor cleaners, and car wash soaps. It is used in lower concentrations with toothpastes, shampoos, and shaving foams. It is an important component in bubble bath formulations for its thickening effect and its ability to create a lather. Research showed that SLS is not carcinogenic when either applied directly to skin or consumed.[4] It has however been shown to irritate the skin of the face with prolonged and constant exposure (more than an hour) in young adults.[5] A clinical study found SLS toothpaste caused a higher frequency of aphthous ulcers than both cocoamidopropyl betaine or a detergent-free paste, on 30 patients with frequent occurrences of such ulcers.[6] A clinical study comparing toothpastes with and without SLS found that it had no significant effect on ulcer patterns.[7] Sodium lauryl sulfate 2 Applications SLS is a highly effective surfactant and is used in any task requiring the removal of oily stains and residues. -

Sodium Lauryl Sulfate
Sodium Lauryl Sulfate Received…………………….……3/7/00 Approval Scientific Review……………..….3/9/00 Biocompatible Provisional Approval IAOMT Board Review………….3/30/00 No Opinion Reevaluation……...……9/01/00, 10/4/05 Periodontal Therapy No Approval……………..…..3/31/00 Explanation of IAOMT position: No explanation is needed. Name of Scientific Review: Sodium Lauryl Sulfate (SLS) Alternative name(s) of Scientific Review: Sodium Laureth Sulfate, Sodium Lauryl Ether Sulfate This Scientific Review is related to: Medicine & Dentistry This Scientific Review is a: Product Do you have a vested financial interest in this Scientific Review? No Purpose of the Scientific Review: To alert to the potential toxicity of SLS in toothpastes and other personal care products. Scientific Review History: SLS has been added as an anionic surfactant or foaming agent in garage floor cleaners, engine degreasers, car wash soaps as well as many toothpastes and shampoos for many years. Briefly describe the Scientific Review: SLS is a major ingredient in many tooth pastes. It should be avoided by all because of its potential toxicity. A specifically, by outline if appropriate, describe the Scientific Review: Because it is cheap, it makes the mixture foam well and it thickens the product appreciably, manufacturers add SLS to most of their toothpastes in more than the 1% recommended maximum addition. Manufacturer(s), Distributor(s), or Publisher: • Manufacturers: Arco Chemical, Dow Chemical, Olin, Eastman Chemical • Manufacturers of these toothpastes use SLS in their products: Aquafresh, Colgate, Crest, Pepsodent, etc. Scientific Literature: • Final Report on the Safety Assessment of Sodium Lauryl Sulfate: Journal of the American College of Toxicology, Vol 2#7, 1083, Mary Ann Liebert, Inc. -

Hazard Communication Chemical Inventory Form
Hazard Communication Chemical Inventory Form ESTIM. CAS STATE QTY. USAGE ROOM SDS DATE OF CHEMICAL NAME COMMON NAME MANUFACTURER NUMBER S,L,G ON HAND PER YEAR CAMPUS NO. DEPARTMENT ? INV. 90wGear Oil NAPA L 5 gal 1 Gal AVC Auto shop Facilities Y 4/2/2018 Acetylene Gas Airgas USA G 33 cu ft 4 cu ft AVC Auto shop Facilities Y 4/2/2018 Antifreeze (Ethylene Glycol) NAPA L 1 gal 2 Gal AVC Auto shop Facilities Y 4/2/2018 Brakleen CRC Industries G 8 cans 12 Cans AVC Auto shop Facilities Y 4/2/2018 CBC Plus Bowl Cleaner ECOLAB L 60 72 AVC Auto shop Facilities Y 4/2/2018 DOT3 Brake Fluid NAPA Mixture L 1 QT 1 QT AVC Auto shop Facilities Y 4/2/2018 Hydraulic Oil NAPA L 10 gal 4 gal AVC Auto shop Facilities Y 4/2/2018 Motor Oil NAPA L 48 Qt 32 Qt AVC Auto shop Facilities Y 4/2/2018 Oxygen Gas Airgas USA G 33 cu ft 10 cu ft AVC Auto shop Facilities Y 4/2/2018 Toluidine Blue 2% Carolina 92-31-9 L 20mL 0mL AVC B112 Science SDS 1/22/2018 Isopropyl Alcohol 2-Propanol, Isopropanol JT Baker(Avantor) 67-63-0 L 75mL 5mL AVC B112 Science SDS 1/22/2018 Ammonium Molybdate ammonium molybdate(VI), tetrahydrate, molybdicFlinn acid 12027-67-7 S 15g 10g AVC B112 Science SDS 1/22/2018 Ammonium Chloride ammonium muriate, sal ammoniac Flinn 12125-02-9 S 40g 5g AVC B112 Science SDS 1/22/2018 Ethylene Glycol Anti-Freeze JT Baker(Avantor) 107-21-1 L 1.25L 10mL AVC B112 Science SDS 1/22/2018 Sodium Bicarbonate Baking Soda VWR (Wards) 144-55-8 S 1,020g 10g AVC B112 Science No 1/22/2018 Sodium Borate Borax VWR 1303-96-4 S 225g 10g AVC B112 Science SDS 1/22/2018 Calcium Metal -

Raw Materials for Cosmetics
Raw materials for cosmetics Sasol Performance Chemicals Raw materials for cosmetics About us Raw materials for cosmetics Register of product groups About us Register of product groups Sasol’s Performance Chemicals business unit markets a broad portfolio 1 Fats and oils/esters 16 of organic and inorganic commodity and speciality chemicals. Our Special esters ................................................... 16 business consists four key business divisions: Organics, Inorganics, Castor oil derivatives ........................................... 16 Wax and PCASG (Phenolics, Carbon, Ammonia and Speciality Gases). Others ........................................................... 16 About 6300 people (incl. employees from Regional Operating Hubs) 2 Fatty alcohols 18 – 21 in offices in 18 countries serve customers around the world with a Single fractions ................................................. 18 multi-faceted portfolio of state-of-the-art chemical products and Blends ........................................................... 20 solutions for a wide range of applications and industries. Monobranched Guerbet alcohols ............................... 20 Our key products include surfactants, surfactant intermediates, fatty alcohols, linear alkyl 3 Petrolatum 22 – 23 benzene (LAB), short-chain linear alpha olefins, ethylene, petrolatum, paraffin waxes, 4 Paraffin waxes 24 – 25 synthetic waxes, cresylic acids, high-quality carbon solutions as well as high-purity and ultra-high-purity alumina. Our speciality gases sub-division supplies -

Sodium Laurilsulfate Used As an Excipient
9 October 2017 EMA/CHMP/351898/2014 corr. 1* Committee for Human Medicinal Products (CHMP) Sodium laurilsulfate used as an excipient Report published in support of the ‘Questions and answers on sodium laurilsulfate used as an excipient in medicinal products for human use’ (EMA/CHMP/606830/2017) * Deletion of the E number. Please see the corrected Annex for further details. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. Reproduction is authorised provided the source is acknowledged. Sodium laurilsulfate used as an excipient Table of contents Executive summary ..................................................................................... 3 Introduction ................................................................................................ 4 Scientific discussion .................................................................................... 4 1. Characteristics ....................................................................................... 4 1.1 Category (function) ............................................................................................. 4 1.2 Properties........................................................................................................... 4 1.3 Use in medicinal products ..................................................................................... 5 1.4 Regulatory -

NON-HAZARDOUS CHEMICALS May Be Disposed of Via Sanitary Sewer Or Solid Waste
NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste (+)-A-TOCOPHEROL ACID SUCCINATE (+,-)-VERAPAMIL, HYDROCHLORIDE 1-AMINOANTHRAQUINONE 1-AMINO-1-CYCLOHEXANECARBOXYLIC ACID 1-BROMOOCTADECANE 1-CARBOXYNAPHTHALENE 1-DECENE 1-HYDROXYANTHRAQUINONE 1-METHYL-4-PHENYL-1,2,5,6-TETRAHYDROPYRIDINE HYDROCHLORIDE 1-NONENE 1-TETRADECENE 1-THIO-B-D-GLUCOSE 1-TRIDECENE 1-UNDECENE 2-ACETAMIDO-1-AZIDO-1,2-DIDEOXY-B-D-GLYCOPYRANOSE 2-ACETAMIDOACRYLIC ACID 2-AMINO-4-CHLOROBENZOTHIAZOLE 2-AMINO-2-(HYDROXY METHYL)-1,3-PROPONEDIOL 2-AMINOBENZOTHIAZOLE 2-AMINOIMIDAZOLE 2-AMINO-5-METHYLBENZENESULFONIC ACID 2-AMINOPURINE 2-ANILINOETHANOL 2-BUTENE-1,4-DIOL 2-CHLOROBENZYLALCOHOL 2-DEOXYCYTIDINE 5-MONOPHOSPHATE 2-DEOXY-D-GLUCOSE 2-DEOXY-D-RIBOSE 2'-DEOXYURIDINE 2'-DEOXYURIDINE 5'-MONOPHOSPHATE 2-HYDROETHYL ACETATE 2-HYDROXY-4-(METHYLTHIO)BUTYRIC ACID 2-METHYLFLUORENE 2-METHYL-2-THIOPSEUDOUREA SULFATE 2-MORPHOLINOETHANESULFONIC ACID 2-NAPHTHOIC ACID 2-OXYGLUTARIC ACID 2-PHENYLPROPIONIC ACID 2-PYRIDINEALDOXIME METHIODIDE 2-STEP CHEMISTRY STEP 1 PART D 2-STEP CHEMISTRY STEP 2 PART A 2-THIOLHISTIDINE 2-THIOPHENECARBOXYLIC ACID 2-THIOPHENECARBOXYLIC HYDRAZIDE 3-ACETYLINDOLE 3-AMINO-1,2,4-TRIAZINE 3-AMINO-L-TYROSINE DIHYDROCHLORIDE MONOHYDRATE 3-CARBETHOXY-2-PIPERIDONE 3-CHLOROCYCLOBUTANONE SOLUTION 3-CHLORO-2-NITROBENZOIC ACID 3-(DIETHYLAMINO)-7-[[P-(DIMETHYLAMINO)PHENYL]AZO]-5-PHENAZINIUM CHLORIDE 3-HYDROXYTROSINE 1 9/26/2005 NON-HAZARDOUS CHEMICALS May Be Disposed Of Via Sanitary Sewer or Solid Waste 3-HYDROXYTYRAMINE HYDROCHLORIDE 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE -

Thieves® Whitening Toothpaste
THIEVES® WHITENING TOOTHPASTE PRODUCT SUMMARY Start your morning on the bright side with the natural power of Thieves® Whitening Toothpaste. Pure and safe ingredients combine to whiten teeth, fight plaque, support healthy gums, and remove stains without damaging enamel. Great for the whole family, Young Living’s exclusive formula is both fluoride free and free of other harsh ingredients, offering you a superior clean you can be confident in. Grin from ear to ear knowing you made a fresh choice with Thieves Whitening Toothpaste! KEY INGREDIENTS Calcium hydroxyapatite, Silica, Perlite, Xylitol, Calcium bicarbonate, Thieves essential oil blend, Spearmint essential oil, Peppermint essential oil, Orange essential oil EXPERIENCE BENEFITS & FEATURES Smile bright with Thieves Whitening Toothpaste. • Whitens and brightens teeth Thieves fans will fall in love with this fresh take on Thieves-infused toothpaste. Fresh Peppermint, • Gently removes surface stains and cleans teeth cool Spearmint, and zesty Orange essential oils • Free from harmful peroxides combine with our spicy Thieves blend to leave your breath EO-fresh all day. Pure and safe whitening • Helps reduce and prevent tartar buildup agents leave you with long-lasting freshness and • Formulated without fluoride, SLS, parabens, a sparkling smile! phthalates, mineral oil, synthetic perfumes or dyes, toxic ingredients, or artificial colors, flavors, or preservatives PRODUCT BACKGROUND • Fights plaque and supports healthy gums Thieves Whitening Toothpaste is one more way Young Living is bringing the power of nature to your • Provides a long-lasting, smooth, fresh feel daily routine: a toothpaste gentle enough for every • Fluoride free and cruelty free day, formulated with essential oils and other natural • Formulated without GMOs ingredients. -

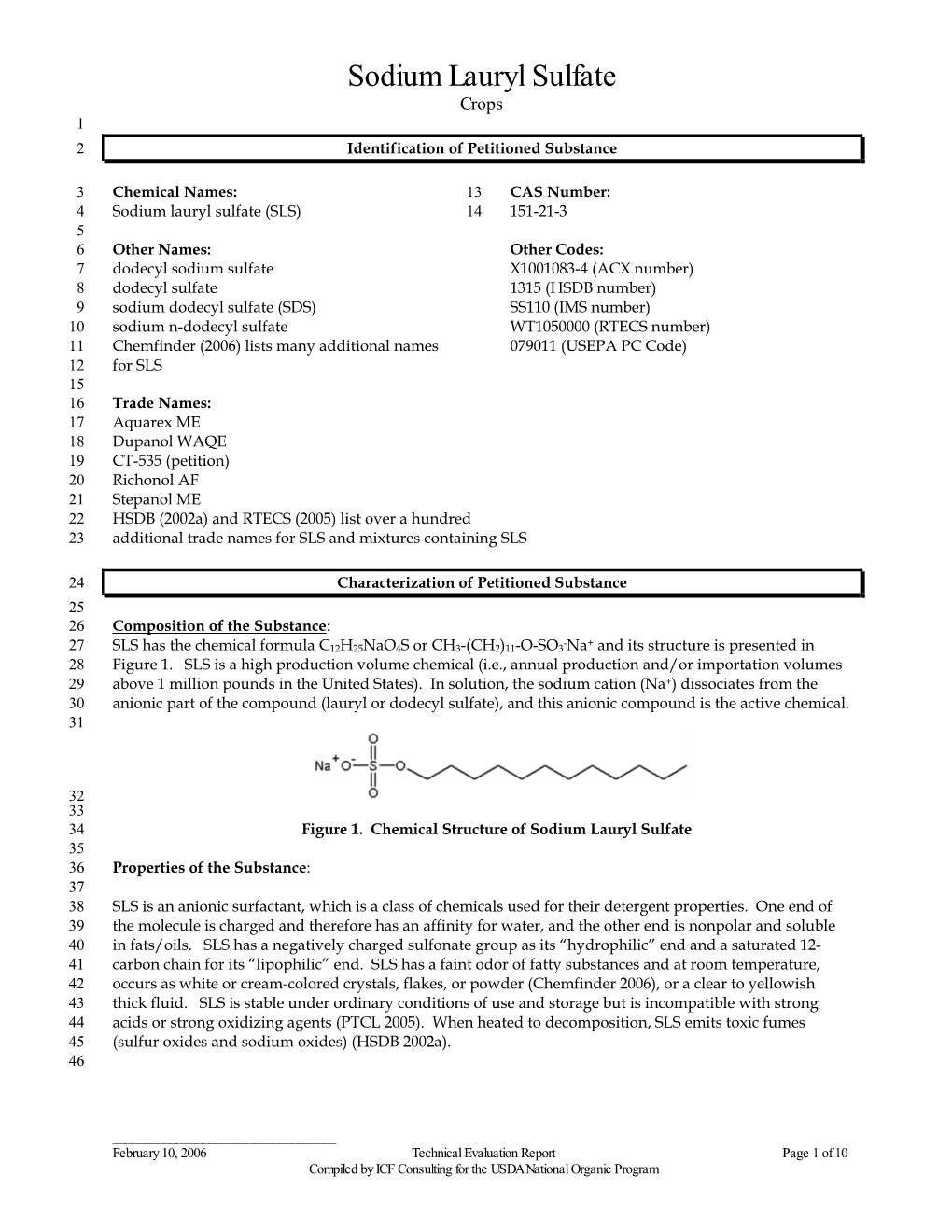
Safety Assessment Dhivacosmetics Dhiva Shampoo
Cosmetic Product Safety Report Product name: Dhiva Shampoo Company name: Dhiva Cosmetics Version: 1 Formula number: Date: October 2016 Part A: Cosmetic Product Safety Information The following information is gathered and managed in the Dhiva Cosmetics product database (the product information file, PIF) under the relevant section. 1. Quantitative and qualitative composition of the cosmetic product, Dhiva shampoo composition (see Appendix A – Quantitative and qualitative composition of the cosmetic product): 2. Physical/chemical characteristics and stability of the cosmetic product: Stability clearance (see Appendix B – Stability summary). 3. Claim support: (no claim on this product). 4. Microbiological quality: Microbiological clearance (see Appendix B – Stability summary). 5. Impurities, traces, information about the packaging material: Packaging clearance (see Appendix B – Stability summary). 6. Normal and reasonably foreseable use: Label specifications (see below 2. Labelled warnings and instructions of use). 7. Exposure to the cosmetic product: (see Appendix C – Exposure assessment) and assessment below 8. Exposure to the substances: MoS calculation (see Appendix D – Margin of Safety calculations). 9. Toxicological profile of the substances (see Appendix E - Toxicological profiles for ingredients). 10. Undesirable effects and serious undesirable effects: Data from reports on (serious) undesireable effects (see Part B: Cosmetic Product Safety Assessment). 11. Information on the cosmetic product: User Test (see Appendix F – User test). Part B: Cosmetic Product Safety Assessment 1. Assessment conclusion The cosmetic product Dhiva Shampoo can be assessed as safe for normal and reasonably foreseeable use in accordance with the European Cosmetics Regulation (EC) No 1223/2009. 2. Labelled warnings and instructions of use The following warnings and instructions of use are mentioned on the packaging material/label of the product: Instructions of use: Massage into wet hair until it lathers. -

Wash N Wax Safety Data Sheet According to the Hazardous Products Regulation (February 11, 2015)
Wash N Wax Safety Data Sheet according to the Hazardous Products Regulation (February 11, 2015) Date of issue: 09/13/2018 Revision date: 09/13/2018 Version: 1.0 SECTION 1: Identification 1.1. Product identifier Product form : Mixture Product name : Wash N Wax Other means of identification : MO18 1.2. Recommended use and restrictions on use Recommended use : Car Shampoo and Car Wax Restrictions on use : Not determined 1.3. Supplier Krown Rust Control 35 MAGNUM DRIVE L0G 1T0 SCHOMBERG - CANADA T (905) 939-8750 1.4. Emergency telephone number Emergency number : (905) 939-8750 SECTION 2: Hazard identification 2.1. Classification of the substance or mixture Classification (GHS-CA) Serious eye damage/eye irritation, Category 2 H319 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS-CA labelling Hazard pictograms (GHS-CA) : Signal word (GHS-CA) : Warning Hazard statements (GHS-CA) : H319 - Causes serious eye irritation. Precautionary statements (GHS-CA) : P264 - Wash hands, forearms and face thoroughly after handling. P280 - Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338 - IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P337+P313 - If eye irritation persists: Get medical advice/attention. 2.3. Other hazards Other hazards not contributing to the : None. classification 2.4. Unknown acute toxicity (GHS-CA) No data available SECTION 3: Composition/information on ingredients 3.1. Substances Not applicable 3.2. Mixtures 09/13/2018 EN (English) Page 1 Wash N Wax Safety Data Sheet according to the Hazardous Products Regulation (February 11, 2015) Name Chemical name / Synonyms Product identifier % Classification (GHS-CA) Poly(oxy-1,2-ethanediyl), .alpha.- Dodecyloxypoly(ethyleneoxy) ethyl (CAS-No.) 9004-82-4 1 - 5 Acute Tox.